Key Points
-
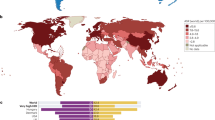
About 25% of lung cancer cases worldwide are not attributable to tobacco smoking. Thus, lung cancer in never smokers is the seventh leading cause of cancer deaths in the world, killing more people every year than pancreatic or prostate cancers.
-
Globally, lung cancer in never smokers demonstrates a marked gender bias, occuring more frequently among women. In particular, there is a high proportion of never smokers in Asian women diagnosed with lung cancer.
-
Although smoking-related carcinogens act on both proximal and distal airways inducing all the major forms of lung cancer, cancers arising in never smokers target the distal airways and favour adenocarcinoma histology.
-
Environmental tobacco smoke (ETS) is a relatively weak carcinogen and can only account for a minority of lung cancers arising in never smokers.
-
Although multiple risk factors, including environmental, hormonal, genetic and viral factors, have been implicated in the pathogenesis of lung cancer in never smokers, no clear-cut dominant factor has emerged that can explain the relatively high incidence of lung cancer in never smokers and the marked geographic differences in gender proportions.
-
Molecular epidemiology studies, in particular of the TP53, KRAS and epidermal growth factor receptor (EGFR) genes, demonstrate strikingly different mutation patterns and frequencies between lung cancers in never smokers and smokers.
-
There are major clinical differences between lung cancers arising in never smokers and smokers and their response to targeted therapies. Indeed, non-smoking status is the strongest clinical predictor of benefit from the EGFR tyrosine kinase inhibitors.
-
The above-mentioned facts strongly suggest that lung cancer arising in never smokers is a disease distinct from the more common tobacco-associated forms of lung cancer.
-
Further efforts are needed to identify the major cause or causes of lung cancers arising in never smokers before successful strategies for prevention, early diagnosis and novel therapies can be implemented.
Abstract
Although most lung cancers are a result of smoking, approximately 25% of lung cancer cases worldwide are not attributable to tobacco use, accounting for over 300,000 deaths each year. Striking differences in the epidemiological, clinical and molecular characteristics of lung cancers arising in never smokers versus smokers have been identified, suggesting that they are separate entities. This Review summarizes our current knowledge of this unique and poorly understood disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Parkin, D. M., Bray, F., Ferlay, J. & Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 (2005).
Brambilla, E., Travis, W. D., Colby, T. V., Corrin, B. & Shimosato, Y. The new World Health Organization classification of lung tumours. Eur. Respir. J. 18, 1059–1068 (2001).
Khuder, S. A. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer 31, 139–148 (2001).
Kreuzer, M., Kreienbrock, L., Muller, K. M., Gerken, M. & Wichmann, E. Histologic types of lung carcinoma and age at onset. Cancer 85, 1958–1965 (1999).
Toh, C. K. et al. Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. J. Clin. Oncol. 24, 2245–2251 (2006).
Dibble, R., Langeburg, W., Bair, S., Ward, J. & Akerley, W. Natural history of non-small cell lung cancer in non-smokers. 23, 7252 (2005).
Lee, C.-T. et al. Characteristics of lung cancer in Korea, 1997. Lung Cancer 30, 15–22 (2000).
Rachtan, J. Smoking, passive smoking and lung cancer cell types among women in Poland. Lung Cancer 35, 129–136 (2002).
Brownson, R. C., Alavanja, M. C., Caporaso, N., Simoes, E. J. & Chang, J. C. Epidemiology and prevention of lung cancer in nonsmokers. Epidemiol. Rev. 20, 218–236 (1998).
Boffetta, P. et al. Multicenter case-control study of exposure to environmental tobacco smoke and lung cancer in Europe. J. Natl Cancer Inst. 90, 1440–1450 (1998).
Du, Y. X. et al. An epidemiological study of risk factors for lung cancer in Guangzhou, China. Lung Cancer 14 (Suppl. 1), S9–S37 (1996).
Prasad, R. et al. Clinicopathological study of bronchogenic carcinoma. Respirology 9, 557–560 (2004).
Radzikowska, E., Glaz, P. & Roszkowski, K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann. Oncol. 13, 1087–1093 (2002).
Kabat, G. C. & Wynder, E. L. Lung cancer in nonsmokers. Cancer 53, 1214–1221 (1984).
Muscat, J. E. & Wynder, E. L. Lung cancer pathology in smokers, ex-smokers and never smokers. Cancer Lett. 88, 1–5 (1995).
Stockwell, H. G. et al. Environmental tobacco smoke and lung cancer risk in nonsmoking women. J. Natl Cancer Inst. 84, 1417–1422 (1992).
Gursel, G., Levent, E., Ozturk, C. & Karalezli, A. Hospital based survey of lung cancer in Turkey, a developing country, where smoking is highly prevalent. Lung Cancer 21, 127–132 (1998).
Ko, Y. C. et al. Risk factors for primary lung cancer among non-smoking women in Taiwan. Int. J. Epidemiol. 26, 24–31 (1997).
Yu, I. T., Chiu, Y. L., Au, J. S., Wong, T. W. & Tang, J. L. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 66, 4961–4967 (2006).
Zhong, L., Goldberg, M. S., Gao, Y. T. & Jin, F. A case-control study of lung cancer and environmental tobacco smoke among nonsmoking women living in Shanghai, China. Cancer Causes Control 10, 607–616 (1999).
Gabrielson, E. Worldwide trends in lung cancer pathology. Respirology 11, 533–538 (2006).
Stratton, K. et al. Clearing the smoke: assessing the science base for tobacco harm reduction. National Academy Press, Washington DC, 2001.
Shields, P. G. Molecular epidemiology of smoking and lung cancer. Oncogene 21, 6870–6876 (2002).
Gray, N. The consequences of the unregulated cigarette. Tob. Control 15, 405–408 (2006).
Liu, N. S. et al. Adenocarcinoma of the lung in young patients: the M. D. Anderson experience. Cancer 88, 1837–1841 (2000).
Hecht, S. S. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 3, 461–469 (2002).
Jemal, A., Chu, K. C. & Tarone, R. E. Recent trends in lung cancer mortality in the United States. J. Natl Cancer Inst. 93, 277–283 (2001).
Jemal, A., Ward, E. & Thun, M. J. Contemporary lung cancer trends among U. S. women. Cancer Epidemiol. Biomarkers Prev. 14, 582–585 (2005).
Bray, F., Tyczynski, J. E. & Parkin, D. M. Going up or coming down? The changing phases of the lung cancer epidemic from 1967 to 1999 in the 15 European Union countries. Euro. J. Cancer 40, 96–125 (2004).
Kuper, H., Boffetta, P. & Adami, H. O. Tobacco use and cancer causation: association by tumour type. J. Intern. Med. 252, 206–224 (2002).
Boffetta, P., Jarvholm, B., Brennan, P. & Nyren, O. Incidence of lung cancer in a large cohort of non-smoking men from Sweden. Int. J. Cancer 94, 591–593 (2001).
Thun, M. J. et al. Lung cancer death rates in lifelong nonsmokers. J. Natl Cancer Inst. 98, 691–699 (2006). The authors correct an important clinical perception, namely that the lung cancer death rate is not higher in female than in male never smokers and shows little evidence of having increased over time in the absence of smoking. Factors that affect the interpretation of lung cancer trends are discussed.
Visbal, A. L. et al. Gender differences in non-small-cell lung cancer survival: an analysis of 4, 618 patients diagnosed between 1997 and 2002. Ann. Thorac. Surg. 78, 209–215 (2004).
Cerfolio, R. J. et al. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest 130, 1796–1802 (2006).
Wakelee, H. A. et al. Lung cancer incidence in never smokers. J. Clin. Oncol. 25, 472–478 (2007).
Koyi, H., Hillerdal, G. & Branden, E. A prospective study of a total material of lung cancer from a county in Sweden 1997–1999: gender, symptoms, type, stage, and smoking habits. Lung Cancer 36, 9–14 (2002).
Brennan, P. et al. High cumulative risk of lung cancer death among smokers and nonsmokers in Central and Eastern Europe. Am. J. Epidemiol. 164, 1233–1241 (2006).
Shimizu, H., Tominaga, S., Nishimura, M. & Urata, A. Comparison of clinico-epidemiological features of lung cancer patients with and without a history of smoking. Jpn J. Clin. Oncol. 14, 595–600 (1984).
Perng, D. W., Perng, R. P., Kuo, B. I. & Chiang, S. C. The variation of cell type distribution in lung cancer: a study of 10, 910 cases at a medical center in Taiwan between 1970 and 1993. Jpn J. Clin. Oncol. 26, 229–233 (1996).
Wakai, K. et al. Tobacco smoking and lung cancer risk: an evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J. Clin. Oncol. 36, 309–324 (2006).
Jindal, S. K. et al. Bronchogenic carcinoma in Northern India. Thorax 37, 343–347 (1982).
Badar, F. et al. Characteristics of lung cancer patients--the Shaukat Khanum Memorial experience. Asian Pac. J. Cancer Prev. 7, 245–248 (2006).
Gazdar, A. F. & Thun, M. J. Lung cancer, smoke exposure, and sex. J. Clin. Oncol. 25, 469–471 (2007).
Subramanian, J. & Govindan, R. Lung cancer in never smokers: a review. J. Clin. Oncol. 25, 561–570 (2007).
Boffetta, P. Human cancer from environmental pollutants: the epidemiological evidence. Mutat. Res. 608, 157–162 (2006).
Alberg, A. J., Brock, M. V. & Samet, J. M. Epidemiology of lung cancer: looking to the future. J. Clin. Oncol. 23, 3175–3185 (2005).
Tobacco smoke and involuntary smoking. IARC Monogr. Eval. Carcinog. Risks Hum. 83, 1–1438 (2004).
Brown, K. in Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders (eds Beyard, S., Jinot, J & Koppikar, A. M.) Chpt 6, 1–29 (Environmental Protection Agency, Washington DC, USA, 1992).
Wu, A. in Health Effects of Exposure to Environmental Tobacco Smoke. (eds Shopland, D., Zeise, L. & Dunn, A.) 282–308 (National Cancer Institute, Bethesda, USA, 1999).
Vineis, P. et al. Tobacco and cancer: recent epidemiological evidence. J. Natl Cancer Inst. 96, 99–106 (2004).
Stayner, L. et al. Lung cancer risk and workplace exposure to environmental tobacco smoke. Am. J. Public Health 97, 545–551 (2007).
U. S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: a Report of the Surgeon General. 2006.
Lubin, J. H. et al. A Joint Analysis of 11 Underground Miners Studies. National Institutes of Health, Bethesda, USA, 1994.
Cross, F. T. Invited commentary: residential radon risks from the perspective of experimental animal studies. Am. J. Epidemiol. 140, 333–339 (1994).
Biological Effects of Ionizing Radiation (BEIR) VI Report: “The Health Effects of Exposure to Indoor Radon”. U. S. National Research Council, 1999.
Darby, S. et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 330, 223 (2005).
Krewski, D. et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J. Toxicol. Environ. Health A 69, 533–597 (2006).
Li, S., Pan, D. & Wang, G. Analysis of polycyclic aromatic hydrocarbons in cooking oil fumes. Arch. Environ. Health 49, 119–122 (1994).
Shields, P. G. et al. Mutagens from heated Chinese and U. S. cooking oils. J. Natl Cancer Inst. 87, 836–841 (1995).
Chiang, T. A. et al. Mutagenicity and polycyclic aromatic hydrocarbon content of fumes from heated cooking oils produced in Taiwan. Mutat. Res. 381, 157–61 (1997).
Yang, S. C., Jenq, S. N., Kang, Z. C. & Lee, H. Identification of benzo[a]pyrene 7, 8-diol 9, 10-epoxide N2-deoxyguanosine in human lung adenocarcinoma cells exposed to cooking oil fumes from frying fish under domestic conditions. Chem. Res. Toxicol. 13, 1046–1050 (2000).
Gao, Y. T. et al. Lung cancer among Chinese women. Int. J. Cancer 40, 604–609 (1987).
Wang, T. J., Zhou, B. S. & Shi, J. P. Lung cancer in nonsmoking Chinese women: a case-control study. Lung Cancer 14 (Suppl. 1), S93–S98 (1996).
Wu-Williams, A. H. et al. Lung cancer among women in north-east China. Br J Cancer 62, 982–7 (1990).
Ko, Y. C. et al. Chinese food cooking and lung cancer in women nonsmokers. Am. J. Epidemiol. 151, 140–147 (2000).
Metayer, C. et al. Cooking oil fumes and risk of lung cancer in women in rural Gansu, China. Lung Cancer 35, 111–117 (2002).
Kleinerman, R. et al. Lung cancer and indoor air pollution in rural china. Ann. Epidemiol. 10, 469 (2000).
Zhong, L., Goldberg, M. S., Gao, Y. T. & Jin, F. Lung cancer and indoor air pollution arising from Chinese-style cooking among nonsmoking women living in Shanghai, China. Epidemiology 10, 488–494 (1999).
Zhou, B. S., Wang, T. J., Guan, P. & Wu, J. M. Indoor air pollution and pulmonary adenocarcinoma among females: a case-control study in Shenyang, China. Oncol. Rep. 7, 1253–1259 (2000).
Seow, A. et al. Fumes from meat cooking and lung cancer risk in Chinese women. Cancer Epidemiol. Biomarkers Prev. 9, 1215–1221 (2000).
Zhao, Y., Wang, S., Aunan, K., Seip, H. M. & Hao, J. Air pollution and lung cancer risks in China--a meta-analysis. Sci. Total Environ. 366, 500–513 (2006).
Mumford, J. L., Helmes, C. T., Lee, X. M., Seidenberg, J. & Nesnow, S. Mouse skin tumorigenicity studies of indoor coal and wood combustion emissions from homes of residents in Xuan Wei, China with high lung cancer mortality. Carcinogenesis 11, 397–403 (1990).
Mumford, J. L. et al. Lung cancer and indoor air pollution in Xuan Wei, China. Science 235, 217–220 (1987).
Xu, Z. Y. et al. Smoking, air pollution, and the high rates of lung cancer in Shenyang, China. J. Natl Cancer Inst. 81, 1800–1806 (1989).
Kleinerman, R. A. et al. Lung cancer and indoor exposure to coal and biomass in rural China. J. Occup. Environ. Med. 44, 338–344 (2002).
Lan, Q., Chapman, R. S., Schreinemachers, D. M., Tian, L. & He, X. Household stove improvement and risk of lung cancer in Xuanwei, China. J. Natl Cancer Inst. 94, 826–835 (2002).
Stabile, L. P. et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor α and β and show biological responses to estrogen. Cancer Res. 62, 2141–2150 (2002).
Mollerup, S., Jorgensen, K., Berge, G. & Haugen, A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer 37, 153–159 (2002).
Fasco, M. J., Hurteau, G. J. & Spivack, S. D. Gender-dependent expression of alpha and β estrogen receptors in human nontumor and tumor lung tissue. Mol. Cell Endocrinol. 188, 125–140 (2002).
Kawai, H. et al. Estrogen receptor α and β are prognostic factors in non-small cell lung cancer. Clin. Cancer Res. 11, 5084–5089 (2005).
Schwartz, A. G. et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin. Cancer Res. 11, 7280–7287 (2005).
Wu, C. T., Chang, Y. L., Shih, J. Y. & Lee, Y. C. The significance of estrogen receptor beta in 301 surgically treated non-small cell lung cancers. J. Thorac. Cardiovasc. Surg. 130, 979–986 (2005).
Pietras, R. J. et al. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids 70, 372–381 (2005).
Hershberger, P. A. et al. Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res. 65, 1598–1605 (2005).
Marquez-Garban, D. C., Chen, H. W., Fishbein, M. C., Goodglick, L. & Pietras, R. J. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 72, 135–143 (2007).
Siegfried, J. M. Women and lung cancer: does oestrogen play a role? Lancet Oncol 2, 506–513 (2001).
Yager, J. D. & Liehr, J. G. Molecular mechanisms of estrogen carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 36, 203–232 (1996).
Cavalieri, E. & Rogan, E. Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: keynote lecture. Ann. NY Acad. Sci. 1089, 286–301 (2006).
Dubey, S., Siegfried, J. M. & Traynor, A. M. Non-small-cell lung cancer and breast carcinoma: chemotherapy and beyond. The Lancet Oncology 7, 416–424 (2006).
Stabile, L. P. et al. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 65, 1459–1470 (2005).
Taioli, E. & Wynder, E. L. Endocrine factors and adenocarcinoma of the lung in women. J. Natl Cancer Inst. 86, 869–870 (1994).
Liu, Y., Inoue, M., Sobue, T. & Tsugane, S. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: a large-scale population-based cohort study. Int. J. Cancer 117, 662–666 (2005).
Ganti, A. K., Sahmoun, A. E., Panwalkar, A. W., Tendulkar, K. K. & Potti, A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J. Clin. Oncol. 24, 59–63 (2006).
Schabath, M. B., Wu, X., Vassilopoulou-Sellin, R., Vaporciyan, A. A. & Spitz, M. R. Hormone replacement therapy and lung cancer risk: a case-control analysis. Clin. Cancer Res. 10, 113–123 (2004).
Kreuzer, M., Gerken, M., Heinrich, J., Kreienbrock, L. & Wichmann, H. E. Hormonal factors and risk of lung cancer among women? Int. J. Epidemiol. 32, 263–271 (2003).
Wu, A. H., Yu, M. C., Thomas, D. C., Pike, M. C. & Henderson, B. E. Personal and family history of lung disease as risk factors for adenocarcinoma of the lung. Cancer Res. 48, 7279–7284 (1988).
Blackman, J. A. et al. Estrogen replacement therapy and risk of lung cancer. Pharmacoepidemiol. Drug Saf. 11, 561–567 (2002).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Matakidou, A., Eisen, T. & Houlston, R. S. Systematic review of the relationship between family history and lung cancer risk. Br. J. Cancer 93, 825–833 (2005).
Cote, M. L., Kardia, S. L., Wenzlaff, A. S., Ruckdeschel, J. C. & Schwartz, A. G. Risk of lung cancer among white and black relatives of individuals with early-onset lung cancer. JAMA 293, 3036–3042 (2005).
Bailey-Wilson, J. E. et al. A major lung cancer susceptibility locus maps to chromosome 6q23–25. Am. J. Hum. Genet. 75, 460–474 (2004). First identification of a major susceptibility locus at 6q23–25 influencing lung cancer risk.
Schwartz, A. G., Prysak, G. M., Bock, C. H. & Cote, M. L. The molecular epidemiology of lung cancer. Carcinogenesis 28, 507–518 (2007).
Hung, R. J. et al. CYP1A1 and GSTM1 genetic polymorphisms and lung cancer risk in Caucasian non-smokers: a pooled analysis. Carcinogenesis 24, 875–882 (2003).
Raimondi, S. et al. Metabolic gene polymorphisms and lung cancer risk in non-smokers. An update of the GSEC study. Mutat. Res. 592, 45–57 (2005).
Zhou, W. et al. Polymorphisms in the DNA repair genes XRCC1 and ERCC2, smoking, and lung cancer risk. Cancer Epidemiol. Biomarkers Prev. 12, 359–365 (2003).
zur Hausen, H. Papillomaviruses in human cancers. Proc. Assoc. Am. Physicians 111, 581–587 (1999).
Cheng, Y. W. et al. The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking Taiwanese women. Cancer Res. 61, 2799–2803 (2001).
Fei, Y. et al. Different human papillomavirus 16/18 infection in Chinese non-small cell lung cancer patients living in Wuhan, China. Jpn J. Clin. Oncol. 36, 274–279 (2006).
Leroux, C. et al. Jaagsiekte Sheep Retrovirus (JSRV): from virus to lung cancer in sheep. Vet. Res. 38, 211–228 (2007).
Liu, G., Zhou, W. & Christiani, D. C. Molecular epidemiology of non-small cell lung cancer. Semin. Respir. Crit. Care Med. 26, 265–272 (2005).
Sharma, S. V., Bell, D. W., Settleman, J. & Haber, D. A. Epidermal growth factor receptor mutations in lung cancer. Nature Rev. Cancer 7, 169–181 (2007). Comprehensive review of a timely and important subject.
Shepherd, F. A. et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 353, 123–32 (2005). First randomized clinical trial demonstrating survival benefit with EGFR TKI inhibitor therapy in previously treated NSCLC.
Thatcher, N. et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366, 1527–1537 (2005).
Herbst, R. S. et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 23, 5892–5899 (2005).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004). First identification (along with reference 116) of mutations in the EGFR gene associated with clinical response to EGFR TKI inhibitors.
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Shigematsu, H. & Gazdar, A. F. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int. J. Cancer 118, 257–262 (2006).
Pham, D. et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J. Clin. Oncol. 24, 1700–1704 (2006).
Kosaka, T. et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 64, 8919–8923 (2004).
Nomura, M. et al. Polymorphisms, mutations and amplification of the EGFR gene in non-small cell lung cancers. PLOS Med. 4, e25 (2007).
Downward, J. Targeting RAS signalling pathways in cancer therapy. Nature Rev. Cancer 3, 11–22 (2003).
Shigematsu, S. et al. Clinical and biological features associated with Epidermal Growth Factor Receptor gene mutations in lung cancers. J. Natl Cancer Inst. 97, 339–346 (2005).
Tam, I. Y. et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin. Cancer Res. 12, 1647–1653 (2006).
Mascaux, C. et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br. J. Cancer 92, 131–139 (2005).
Pao, W. et al. KRAS Mutations and primary resistance of lung adenocarcinomas to Gefitinib or Erlotinib. PLOS Med. 2, e17 (2005).
Johnson, L. et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410, 1111–1116 (2001).
Politi, K. et al. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 20, 1496–1510 (2006).
Wistuba, I. & Gazdar, A. F. Lung cancer preneoplasia. Annu. Rev. Pathol. Mech. Dis. 1, 331–348 (2006).
Sengupta, S. & Harris, C. C. p53: traffic cop at the crossroads of DNA repair and recombination. Nature Rev. Mol. Cell Biol. 6, 44–55 (2005).
Vahakangas, K. H. et al. p53 and K-ras mutations in lung cancers from former and never-smoking women. Cancer Res. 61, 4350–4356 (2001).
Le Calvez, F. et al. TP53 and KRAS mutation load and types in lung cancers in relation to tobacco smoke: distinct patterns in never, former, and current smokers. Cancer Res. 65, 5076–5083 (2005). Modest sized but well conducted study on the relationship of TP53 mutation pattern, smoking and lung cancer.
Denissenko, M. F., Chen, J. X., Tang, M. S. & Pfeifer, G. P. Cytosine methylation determines hot spots of DNA damage in the human P53 gene. Proc. Natl Acad. Sci. USA 94, 3893–3898 (1997). Important early work demonstrating the targeting of CpG sites by tobacco carcinogen adducts.
Sozzi, G. et al. Association between cigarette smoking and FHIT gene alterations in lung cancer. Cancer Res. 57, 2121–2123 (1997).
Marchetti, A. et al. Genetic analysis of lung tumours of non-smoking subjects: p53 gene mutations are constantly associated with loss of heterozygosity at the FHIT locus. Br. J. Cancer 78, 73–78 (1998).
Takeuchi, T. et al. Expression profile-defined classification of lung adenocarcinoma shows close relationship with underlying major genetic changes and clinicopathologic behaviors. J. Clin. Oncol. 24, 1679–1688 (2006).
Lam, D. C. et al. Establishment and expression profiling of new lung cancer cell lines from Chinese smokers and lifetime never-smokers. J. Thorac. Oncol. 1, 932–942 (2006).
Dutu, T. et al. Differential expression of biomarkers in lung adenocarcinoma: a comparative study between smokers and never-smokers. Ann. Oncol. 16, 1906–1914 (2005).
Koo, L. C., Ho, J. H. & Lee, N. An analysis of some risk factors for lung cancer in Hong Kong. Int. J. Cancer 35, 149–155 (1985).
Nordquist, L. T., Simon, G. R., Cantor, A., Alberts, W. M. & Bepler, G. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest 126, 347–351 (2004).
Tammemagi, C. M., Neslund-Dudas, C., Simoff, M. & Kvale, P. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest 125, 27–37 (2004).
Zell, J. A., Ou, S. H., Ziogas, A. & Anton-Culver, H. Epidemiology of bronchioloalveolar carcinoma: improvement in survival after release of the 1999 WHO classification of lung tumors. J. Clin. Oncol. 23, 8396–8405 (2005).
Toh, C. K. et al. The impact of smoking status on the behavior and survival outcome of patients with advanced non-small cell lung cancer: a retrospective analysis. Chest 126, 1750–1756 (2004).
Tsao, A. S., Liu, D., Lee, J. J., Spitz, M. & Hong, W. K. Smoking affects treatment outcome in patients with advanced nonsmall cell lung cancer. Cancer 106, 2428–2436 (2006).
Miller, V. A. et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J. Clin. Oncol. 22, 1103–1109 (2004).
Lim, S. T. et al. Gefitinib is more effective in never-smokers with non-small-cell lung cancer: experience among Asian patients. Br. J. Cancer 93, 23–28 (2005).
Gazdar, A. F. DNA repair and survival in lung cancer--the two faces of Janus. N. Engl. J. Med. 356, 771–773 (2007).
Kim, C. F. et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121, 823–835 (2005).
Maeda, Y., Dave, V. & Whitsett, J. A. Transcriptional control of lung morphogenesis. Physiol. Rev. 87, 219–244 (2007).
Yatabe, Y. Epidermal growth factor receptor mutations in lung cancers. Pathol. Int. 57, 233–244 (2007).
Yatabe, Y., Mitsudomi, T. & Takahashi, T. TTF-1 expression in pulmonary adenocarcinomas. Am. J. Surg. Pathol. 26, 767–773 (2002).
Yatabe, Y., Kosaka, T., Takahashi, T. & Mitsudomi, T. EGFR mutation is specific for terminal respiratory unit type adenocarcinoma. Am. J. Surg. Pathol. 29, 633–639 (2005). Part hypothesis, part fact that EGFR mutations target the stem cells of the peripheral airways.
Giangreco, A., Reynolds, S. D. & Stripp, B. R. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am. J. Pathol. 161, 173–182 (2002).
Eberhard, D. A. et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J. Clin. Oncol. 23, 5900–5909 (2005).
Soung, Y. H. et al. Mutational analysis of EGFR and K-RAS genes in lung adenocarcinomas. Virchows Arch. 446, 483–488 (2005).
Blons, H. et al. Epidermal growth factor receptor mutation in lung cancer are linked to bronchioloalveolar differentiation. Am. J. Surg. Pathol. 30, 1309–1315 (2006).
Ahrendt, S. A. et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer 92, 1525–1530 (2001).
Toyooka, S., Tsuda, T. & Gazdar, A. F. The TP53 gene, tobacco exposure, and lung cancer. Hum. Mutat. 21, 229–239 (2003).
Lissowska, J. et al. Lung cancer and indoor pollution from heating and cooking with solid fuels: the IARC international multicentre case-control study in Eastern/Central Europe and the United Kingdom. Am. J. Epidemiol. 162, 326–333 (2005).
Ramanakumar, A. V., Parent, M. E. & Siemiatycki, J. Risk of lung cancer from residential heating and cooking fuels in Montreal, Canada. Am. J. Epidemiol. 165, 634–642 (2007).
Hung, R. J., Hall, J., Brennan, P. & Boffetta, P. Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review. Am. J. Epidemiol. 162, 925–942 (2005).
Hung, R. J. et al. Large-scale investigation of base excision repair genetic polymorphisms and lung cancer risk in a multicenter study. J. Natl Cancer Inst. 97, 567–576 (2005).
Toyooka, S. et al. Mutational and epigenetic evidence for independent pathways for lung adenocarcinomas arising in smokers and never smokers. Cancer Res. 66, 1371–1375 (2006).
Divine, K. K. et al. Multiplicity of abnormal promoter methylation in lung adenocarcinomas from smokers and never smokers. Int. J. Cancer 114, 400–405 (2005).
Acknowledgements
We thank Xian-Jin Xie for his help with the statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Lung cancer
-
A malignant tumour arising from the cells of the respiratory epithelium. By definition, tumours arising from the non epithelial cells (sarcomas and lymphomas) or the mesothelial lining (mesotheliomas) of the lung are excluded.
- Small cell carcinoma of the lung
-
(SCLC) A highly malignant type of carcinoma consisting of small cells expressing neuroendocrine features. Almost all cases arise in ever smokers.
- Squamous cell carcinoma
-
Tumours that arise from multilayered squamous lining cells. Squamous cells are not normally present in the respiratory epithelium, but may arise from glandular or secretory cells by metaplastic change as a result of exposure to tobacco products, inflammation, irritation etc.
- Adenocarcinoma
-
A tumour arising from cells that have glandular or secretory properties.
- Nicotine
-
An alkaloid compound found in certain plants, especially in tobacco. Nicotine constitutes 0.3 to 5% of the tobacco plant by dry weight, with biosynthesis taking place in the roots and accumulating in the leaves. It acts as a stimulant and is one of the main factors responsible for the dependence-forming properties of tobacco smoking.
- Environmental tobacco smoke
-
(ETS). A combination of the smoke that comes from the burning of a tobacco product and smoke that is exhaled by smokers (second-hand smoke). Inhaling ETS is called involuntary or passive smoking.
- Nitrosamines
-
Any of a class of organic compounds with the general formula R2NNO or RNHNO, present in various foods, tobacco and other products. Many nitrosamines are carcinogenic.
- Polycyclic aromatic hydrocarbons
-
(PAHs). A group of over 100 different stable organic molecules made up of only carbon and hydrogen. They are large, flat molecules built of a collection of fused benzene-like rings. They are formed during the incomplete burning of coal, oil and gas, garbage, or other organic substances like tobacco or charbroiled meat.
- DNA adducts
-
Altered forms of DNA that occur as the result of exposure to carcinogens (in the case of smokers these would be the carcinogens present in cigarette smoke). A DNA adduct may have several possible outcomes: it may lead to cell death by apoptosis; it may be repaired, resulting in a return to the original DNA structure; or it may be mis-repaired, resulting in a mutation.
- Base excision repair
-
A DNA repair pathway that operates on small DNA lesions such as oxidized or reduced bases, fragmented or non-bulky adducts, or those produced by methylating agents; includes XRCC and OGG1.
- DNA double-strand break repair
-
DNA double-strand breaks (DSBs) occur as intermediates in DNA metabolic functions and from exogenous agents such as ionizing radiation; two pathways for DSB repair include homologous recombination and non-homologous end-joining.
- Mismatch repair
-
Corrects DNA replication errors (base–base or insertion/deletion mismatches) caused by DNA polymerase errors.
- Bronchioloalveolar carcinoma
-
(BAC). As strictly defined, a non-invasive form of peripherally arising adenocarcinoma. However, often incorrectly applied to adenocarcinomas of mixed histology that have a prominent bronchioloalveolar component.
- Missense mutation
-
A mutation that causes an amino acid substitution.
- CpG island
-
A part of the genomic DNA in which the frequency of the cytosine (C)–guanine (G) sequence is higher than predicted. The p indicates that C and G are connected by a phosphodiester bond. Methylation only occurs at the C nucleotide of CpG sites. CpG islands are located around the promoters of about half of all genes.
Rights and permissions
About this article
Cite this article
Sun, S., Schiller, J. & Gazdar, A. Lung cancer in never smokers — a different disease. Nat Rev Cancer 7, 778–790 (2007). https://doi.org/10.1038/nrc2190
Issue Date:
DOI: https://doi.org/10.1038/nrc2190
This article is cited by
-
Assessment of air pollution emitted during cooking using biomass and cleaner fuels in the Shiselweni region of Eswatini (Swaziland)
Clean Technologies and Environmental Policy (2024)
-
New insights into the biology and development of lung cancer in never smokers—implications for early detection and treatment
Journal of Translational Medicine (2023)
-
Lung cancer in the emergency department
Emergency Cancer Care (2023)
-
TP53 mutation prevalence in normal airway epithelium as a biomarker for lung cancer risk
BMC Cancer (2023)
-
Incidence trends and spatial distributions of lung adenocarcinoma and squamous cell carcinoma in Taiwan
Scientific Reports (2023)