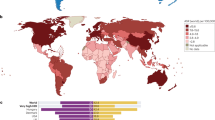
Abstract
Lung cancer is the most common cause of cancer-related deaths globally. Although smoking-related lung cancers continue to account for the majority of diagnoses, smoking rates have been decreasing for several decades. Lung cancer in individuals who have never smoked (LCINS) is estimated to be the fifth most common cause of cancer-related deaths worldwide in 2023, preferentially occurring in women and Asian populations. As smoking rates continue to decline, understanding the aetiology and features of this disease, which necessitate unique diagnostic and treatment paradigms, will be imperative. New data have provided important insights into the molecular and genomic characteristics of LCINS, which are distinct from those of smoking-associated lung cancers and directly affect treatment decisions and outcomes. Herein, we review the emerging data regarding the aetiology and features of LCINS, particularly the genetic and environmental underpinnings of this disease as well as their implications for treatment. In addition, we outline the unique diagnostic and therapeutic paradigms of LCINS and discuss future directions in identifying individuals at high risk of this disease for potential screening efforts.
Key points
-
The global incidence of lung cancer is decreasing in parallel with declining smoking rates in developed countries; however, the incidence of lung cancer in individuals who have never smoked (LCINS) is stable or increasing.
-
LCINS is the eighth leading cause of cancer-related mortality in the USA and the fifth most common cause of cancer-related deaths worldwide.
-
LCINS has histological and epidemiological distinctions from smoking-related lung cancers, occurring almost exclusively as adenocarcinomas and most commonly in women and individuals of Asian ancestry.
-
LCINS are highly enriched for targetable oncogenic alterations, have low tumour mutational burden and low rates of PD-L1 positivity, and lack mutational signatures, even in patients who report passive, secondhand smoke exposure.
-
LCINS development probably involves interactions between genetic risk, mediated by common and rare germline variants, and environmental exposures, including air pollution and particulate matter, with potential opportunities for broader lung cancer screening.
-
In the era of precision oncology, the biological underpinnings of LCINS necessitate unique diagnostic and treatment paradigms and warrant consideration of this disease as an important and distinct clinical entity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Cornelius, M. E. et al. Tobacco product use among adults - United States, 2021. MMWR Morb. Mortal. Wkly Rep. 72, 475–483 (2023).
The American Lung Association. Overall Tobacco Trends. Analysis of Centers for Disease Control and Prevention, National Center for Health Statistics. National Health Interview Survey 1965-2018. lung.org, https://www.lung.org/research/trends-in-lung-disease/tobacco-trends-brief/overall-tobacco-trends (2019).
Jeon, J. et al. Smoking and lung cancer mortality in the United States from 2015 to 2065: a comparative modeling approach. Ann. Intern. Med. 169, 684–693 (2018).
Pelosof, L. et al. Proportion of never-smoker non-small cell lung cancer patients at three diverse institutions. J. Natl Cancer Inst. 109, djw295 (2017).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Devarakonda, S. et al. Genomic profiling of lung adenocarcinoma in never-smokers. J. Clin. Oncol. 39, 3747–3758 (2021).
Ha, S. Y. et al. Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often involving EGFR. Oncotarget 6, 5465–5474 (2015).
International Association for the Study of Lung Cancer. IASLC Language Guide https://www.iaslc.org/IASLCLanguageGuide (2021).
Pham, D. et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J. Clin. Oncol. 24, 1700–1704 (2006).
Thu, K. L. et al. Lung adenocarcinoma of never smokers and smokers harbor differential regions of genetic alteration and exhibit different levels of genomic instability. PLoS ONE 7, e33003 (2012).
Brown, K. F. et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br. J. Cancer 118, 1130–1141 (2018).
Islami, F. et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 68, 31–54 (2018).
Parkin, D. M. 2. Tobacco-attributable cancer burden in the UK in 2010. Br. J. Cancer 105, S6–S13 (2011).
Islami, F. et al. Cancer deaths and cases attributable to lifestyle factors and infections in China, 2013. Ann. Oncol. 28, 2567–2574 (2017).
Siegel, D. A., Fedewa, S. A., Henley, S. J., Pollack, L. A. & Jemal, A. Proportion of never smokers among men and women with lung cancer in 7 US States. JAMA Oncol. 7, 302–304 (2021).
Thun, M. J. et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 5, e185 (2008).
Gitlitz, B. J. et al. The genomics of young lung cancer: comprehensive tissue genomic analysis in patients under 40 with lung cancer. JTO Clin. Res. Rep. 2, 100194 (2021).
Shaw, A. T. et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. 27, 4247–4253 (2009).
Wong, D. W. et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 115, 1723–1733 (2009).
Kim, H. et al. A comprehensive comparative analysis of the histomorphological features of ALK-rearranged lung adenocarcinoma based on driver oncogene mutations: frequent expression of epithelial-mesenchymal transition markers than other genotype. PLoS ONE 8, e76999 (2013).
Gainor, J. F. et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis. Oncol. 2017, PO.17.00063 (2017).
Lin, C., Wang, S., Xie, W., Chang, J. & Gan, Y. The RET fusion gene and its correlation with demographic and clinicopathological features of non-small cell lung cancer: a meta-analysis. Cancer Biol. Ther. 16, 1019–1028 (2015).
Hess, L. M., Han, Y., Zhu, Y. E., Bhandari, N. R. & Sireci, A. Characteristics and outcomes of patients with RET-fusion positive non-small lung cancer in real-world practice in the United States. BMC Cancer 21, 28 (2021).
Mazieres, J. et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J. Clin. Oncol. 33, 992–999 (2015).
Park, S. et al. Characteristics and outcome of ROS1-positive non-small cell lung cancer patients in routine clinical practice. J. Thorac. Oncol. 13, 1373–1382 (2018).
Chen, Y. F. et al. Clinical and the prognostic characteristics of lung adenocarcinoma patients with ROS1 fusion in comparison with other driver mutations in East Asian populations. J. Thorac. Oncol. 9, 1171–1179 (2014).
Farago, A. F. et al. Clinicopathologic features of non-small-cell lung cancer harboring an NTRK gene fusion.JCO Precis. Oncol. 2018, PO.18.00037 (2018).
Leiter, A., Veluswamy, R. R. & Wisnivesky, J. P. The global burden of lung cancer: current status and future trends. Nat. Rev. Clin. Oncol. 20, 624–639 (2023).
United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. National Program of Cancer Registries and Surveillance, Epidemiology and End Results Program SEER*Stat Database: NPCR and SEER Incidence — U.S. Cancer Statistics Public Use Research Database, 2021 Submission (2001-2019) www.cdc.gov/cancer/uscs/public-use (2022).
Walters, K. A., Li, Y., Tiwari, R. C. & Zou, Z. A weighted-least-squares estimation approach to comparing trends in age-adjusted cancer rates across overlapping regions. J. Data Sci. 8, 631–644 (2011).
National Cancer Institute Surveillance Research Program. SEER*Stat software version 8.4.2. — August 14, 2023 https://seer.cancer.gov/seerstat/ (2023).
Harris, J. E. Cigarette smoking among successive birth cohorts of men and women in the United States during 1900-80. J. Natl Cancer Inst. 71, 473–479 (1983).
Jemal, A., Ma, J., Rosenberg, P. S., Siegel, R. & Anderson, W. F. Increasing lung cancer death rates among young women in southern and midwestern States. J. Clin. Oncol. 30, 2739–2744 (2012).
Jemal, A. et al. Higher lung cancer incidence in young women than young men in the United States. N. Engl. J. Med. 378, 1999–2009 (2018).
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking — 50 Years of Progress: A Report of the Surgeon General (Centers for Disease Control and Prevention (US), 2014).
Lewis, D. R., Check, D. P., Caporaso, N. E., Travis, W. D. & Devesa, S. S. US lung cancer trends by histologic type. Cancer 120, 2883–2892 (2014).
Pesch, B. et al. Cigarette smoking and lung cancer — relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int. J. Cancer 131, 1210–1219 (2012).
Agudo, A. et al. Impact of cigarette smoking on cancer risk in the European prospective investigation into cancer and nutrition study. J. Clin. Oncol. 30, 4550–4557 (2012).
Alexandrov, L. B. et al. Mutational signatures associated with tobacco smoking in human cancer. Science 354, 618–622 (2016).
Ogino, A. et al. Genomic and pathological heterogeneity in clinically diagnosed small cell lung cancer in never/light smokers identifies therapeutically targetable alterations. Mol. Oncol. 15, 27–42 (2021).
Maeda, R. et al. Influence of cigarette smoking on histological subtypes of stage I lung adenocarcinoma. J. Thorac. Oncol. 6, 743–750 (2011).
Sugawara, H. et al. Adenocarcinoma in situ and minimally invasive adenocarcinoma in lungs of smokers: image feature differences from those in lungs of non-smokers. BMC Med. Imaging 21, 172 (2021).
Inamura, K. et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod. Pathol. 22, 508–515 (2009).
Rodig, S. J. et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the Western population. Clin. Cancer Res. 15, 5216–5223 (2009).
Yoshida, A. et al. Frequent ALK rearrangement and TTF-1/p63 co-expression in lung adenocarcinoma with signet-ring cell component. Lung Cancer 72, 309–315 (2011).
Yoshida, A. et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am. J. Surg. Pathol. 35, 1226–1234 (2011).
Popat, S. et al. ALK translocation is associated with ALK immunoreactivity and extensive signet-ring morphology in primary lung adenocarcinoma. Lung Cancer 75, 300–305 (2012).
Nishino, M. et al. Histologic and cytomorphologic features of ALK-rearranged lung adenocarcinomas. Mod. Pathol. 25, 1462–1472 (2012).
Pan, Y. et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer 84, 121–126 (2014).
Nakada, T. et al. Imaging characteristics in ALK fusion-positive lung adenocarcinomas by using HRCT. Ann. Thorac. Cardiovasc. Surg. 21, 102–108 (2015).
Yoon, H. J. et al. Decoding tumor phenotypes for ALK, ROS1, and RET fusions in lung adenocarcinoma using a radiomics approach. Medicine 94, e1753 (2015).
Han, X. et al. CT features associated with EGFR mutations and ALK positivity in patients with multiple primary lung adenocarcinomas. Cancer Imaging 20, 51 (2020).
Yamamoto, S. et al. ALK molecular phenotype in non-small cell lung cancer: CT radiogenomic characterization. Radiology 272, 568–576 (2014).
Glynn, C., Zakowski, M. F. & Ginsberg, M. S. Are there imaging characteristics associated with epidermal growth factor receptor and KRAS mutations in patients with adenocarcinoma of the lung with bronchioloalveolar features? J. Thorac. Oncol. 5, 344–348 (2010).
Pinheiro, G. et al. Identifying relationships between imaging phenotypes and lung cancer-related mutation status: EGFR and KRAS. Sci. Rep. 10, 3625 (2020).
Rossi, G. et al. Radiomic detection of EGFR mutations in NSCLC. Cancer Res. 81, 724–731 (2021).
Nishino, M. et al. Tumor volume decrease at 8 weeks is associated with longer survival in EGFR-mutant advanced non-small-cell lung cancer patients treated with EGFR TKI. J. Thorac. Oncol. 8, 1059–1068 (2013).
Nishino, M. et al. Volumetric tumor growth in advanced non-small cell lung cancer patients with EGFR mutations during EGFR-tyrosine kinase inhibitor therapy: developing criteria to continue therapy beyond RECIST progression. Cancer 119, 3761–3768 (2013).
Nishino, M. et al. Volumetric tumor response and progression in EGFR-mutant NSCLC patients treated with erlotinib or gefitinib. Acad. Radiol. 23, 329–336 (2016).
Nishino, M. Tumor response assessment for precision cancer therapy: response evaluation criteria in solid tumors and beyond. Am. Soc. Clin. Oncol. Educ. Book 38, 1019–1029 (2018).
Park, H., Sholl, L. M., Hatabu, H., Awad, M. M. & Nishino, M. Imaging of precision therapy for lung cancer: current state of the art. Radiology 293, 15–29 (2019).
Nishino, M. et al. Tumor growth rate after nadir is associated with survival in patients with egfr-mutant non-small-cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitor. JCO Precis. Oncol. 5, 1603–1610 (2021).
Shiri, I. et al. Next-generation radiogenomics sequencing for prediction of EGFR and KRAS mutation status in NSCLC patients using multimodal imaging and machine learning algorithms. Mol. Imaging Biol. 22, 1132–1148 (2020).
Wang, G. et al. Radiomics signature of brain metastasis: prediction of EGFR mutation status. Eur. Radiol. 31, 4538–4547 (2021).
Schuette, W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer 45, S253–257 (2004).
Heon, S. et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin. Cancer Res. 16, 5873–5882 (2010).
Costa, D. B. et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J. Clin. Oncol. 33, 1881–1888 (2015).
Zhang, I., Zaorsky, N. G., Palmer, J. D., Mehra, R. & Lu, B. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol. 16, e510–521 (2015).
Johung, K. L. et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J. Clin. Oncol. 34, 123–129 (2016).
Soria, J. C. et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 389, 917–929 (2017).
Peters, S. et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 377, 829–838 (2017).
Shin, D. Y. et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J. Thorac. Oncol. 9, 195–199 (2014).
Rangachari, D. et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 88, 108–111 (2015).
Patil, T. et al. The incidence of brain metastases in stage IV ROS1-rearranged non-small cell lung cancer and rate of central nervous system progression on crizotinib. J. Thorac. Oncol. 13, 1717–1726 (2018).
Reungwetwattana, T. et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J. Clin. Oncol. https://doi.org/10.1200/JCO.2018.78.3118 (2018).
Zhou, F. & Zhou, C. Lung cancer in never smokers-the East Asian experience. Transl. Lung Cancer Res. 7, 450–463 (2018).
Zhang, T. et al. Genomic and evolutionary classification of lung cancer in never smokers. Nat. Genet. 53, 1348–1359 (2021).
Choudhury, N. J. et al. The GENIE BPC NSCLC cohort: a real-world repository integrating standardized clinical and genomic data for 1,846 patients with non-small cell lung cancer. Clin. Cancer Res. 29, 3418–3428 (2023).
Harada, G., Yang, S. R., Cocco, E. & Drilon, A. Rare molecular subtypes of lung cancer. Nat. Rev. Clin. Oncol. 20, 229–249 (2023).
Lee, B., Lee, T., Lee, S. H., Choi, Y. L. & Han, J. Clinicopathologic characteristics of EGFR, KRAS, and ALK alterations in 6,595 lung cancers. Oncotarget 7, 23874–23884 (2016).
Dogan, S. et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin. Cancer Res. 18, 6169–6177 (2012).
Lee, Y. J. et al. Dose effect of cigarette smoking on frequency and spectrum of epidermal growth factor receptor gene mutations in Korean patients with non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 136, 1937–1944 (2010).
Beau-Faller, M. et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann. Oncol. 25, 126–131 (2014).
Oxnard, G. R. et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J. Thorac. Oncol. 8, 179–184 (2013).
Arcila, M. E. et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol. Cancer Ther. 12, 220–229 (2013).
Burnett, H. et al. Epidemiological and clinical burden of EGFR exon 20 insertion in advanced non-small cell lung cancer: a systematic literature review. PLoS ONE 16, e0247620 (2021).
Konduri, K. et al. EGFR fusions as novel therapeutic targets in lung cancer. Cancer Discov. 6, 601–611 (2016).
Zhu, Y. C. et al. EGFR-RAD51 fusion variant in lung adenocarcinoma and response to erlotinib: a case report. Lung Cancer 115, 131–134 (2018).
Guan, Y. et al. Effectiveness of EGFR-TKIs in a patient with lung adenocarcinoma harboring an EGFR-RAD51 fusion. Oncologist 24, 1027–1030 (2019).
Yamaguchi, N. et al. Smoking status and self-reported race affect the frequency of clinically relevant oncogenic alterations in non-small-cell lung cancers at a United States-based academic medical practice. Lung Cancer 82, 31–37 (2013).
Couraud, S. et al. BioCAST/IFCT-1002: epidemiological and molecular features of lung cancer in never-smokers. Eur. Respir. J. 45, 1403–1414 (2015).
Solomon, B., Varella-Garcia, M. & Camidge, D. R. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J. Thorac. Oncol. 4, 1450–1454 (2009).
Chang, G. C. et al. ALK variants, PD-L1 expression, and their association with outcomes in ALK-positive NSCLC patients. Sci. Rep. 10, 21063 (2020).
Inamura, K. et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J. Thorac. Oncol. 3, 13–17 (2008).
Boland, J. M. et al. Anaplastic lymphoma kinase immunoreactivity correlates with ALK gene rearrangement and transcriptional up-regulation in non-small cell lung carcinomas. Hum. Pathol. 40, 1152–1158 (2009).
Izumi, H. et al. The CLIP1-LTK fusion is an oncogenic driver in non-small-cell lung cancer. Nature 600, 319–323 (2021).
Cooper, A. J., Sequist, L. V., Johnson, T. W. & Lin, J. J. LTK fusions: a new target emerges in non-small cell lung cancer. Cancer Cell 40, 23–25 (2022).
Cadranel, J. et al. Therapeutic potential of afatinib in NRG1 fusion-driven solid tumors: a case series. Oncologist 26, 7–16 (2021).
Liu, S. V., Minasi, L. A. E., Herpers, M. & Frohn, C. Efficacy of afatinib in patients with advanced/metastatic solid tumors harboring NRG1 gene fusions: a novel, prospective real-world outcomes study based on single-patient protocol data. J. Clin. Oncol. 40, TPS3180 (2022).
Riely, G. J. et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin. Cancer Res. 14, 5731–5734 (2008).
Kucab, J. E. et al. A compendium of mutational signatures of environmental agents. Cell 177, 821–836.e16 (2019).
Pich, O. et al. The mutational footprints of cancer therapies. Nat. Genet. 51, 1732–1740 (2019).
Ricciuti, B. et al. Dissecting the clinicopathologic, genomic, and immunophenotypic correlates of KRAS(G12D)-mutated non-small-cell lung cancer. Ann. Oncol. 33, 1029–1040 (2022).
Frampton, G. M. et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 5, 850–859 (2015).
Schrock, A. B. et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J. Thorac. Oncol. 11, 1493–1502 (2016).
Tong, J. H. et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin. Cancer Res. 22, 3048–3056 (2016).
Awad, M. M. et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent met genomic amplification and c-Met overexpression. J. Clin. Oncol. 34, 721–730 (2016).
Schildhaus, H. U. et al. MET amplification status in therapy-naive adeno- and squamous cell carcinomas of the lung. Clin. Cancer Res. 21, 907–915 (2015).
Okuda, K., Sasaki, H., Yukiue, H., Yano, M. & Fujii, Y. Met gene copy number predicts the prognosis for completely resected non-small cell lung cancer. Cancer Sci. 99, 2280–2285 (2008).
Onozato, R. et al. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J. Thorac. Oncol. 4, 5–11 (2009).
Wolf, J. et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N. Engl. J. Med. 383, 944–957 (2020).
Noonan, S. A. et al. Identifying the appropriate FISH criteria for defining MET copy number-driven lung adenocarcinoma through oncogene overlap analysis. J. Thorac. Oncol. 11, 1293–1304 (2016).
Le, X. et al. Tepotinib in patients with non-small cell lung cancer with high-level MET amplification detected by liquid biopsy: VISION Cohort B. Cell Rep. Med. 4, 101280 (2023).
Yu, H. A. et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 19, 2240–2247 (2013).
Chabon, J. J. et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat. Commun. 7, 11815 (2016).
Dagogo-Jack, I. et al. MET alterations are a recurring and actionable resistance mechanism in ALK-positive lung cancer. Clin. Cancer Res. 26, 2535–2545 (2020).
Kron, A. et al. Genetic heterogeneity of MET-aberrant NSCLC and its impact on the outcome of immunotherapy. J. Thorac. Oncol. 16, 572–582 (2021).
Plenker, D. et al. Structural alterations of MET trigger response to MET kinase inhibition in lung adenocarcinoma patients. Clin. Cancer Res. 24, 1337–1343 (2018).
Cho, J. H. et al. KIF5B-MET gene rearrangement with robust antitumor activity in response to crizotinib in lung adenocarcinoma. J. Thorac. Oncol. 13, e29–e31 (2018).
Liu, L. F., Deng, J. Y., Lizaso, A., Lin, J. & Sun, S. Effective response to crizotinib of concurrent KIF5B-MET and MET-CDR2-rearranged non-small cell lung cancer: a case report. World J. Clin. Cases 10, 2529–2536 (2022).
Stephens, P. et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 431, 525–526 (2004).
Barlesi, F. et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 387, 1415–1426 (2016).
Arcila, M. E. et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin. Cancer Res. 18, 4910–4918 (2012).
Zhao, J. & Xia, Y. Targeting HER2 alterations in non-small-cell lung cancer: a comprehensive review. JCO Precis. Oncol. 4, 411–425 (2020).
Ou, S. I. et al. HER2 transmembrane domain (TMD) mutations (V659/G660) that stabilize homo- and heterodimerization are rare oncogenic drivers in lung adenocarcinoma that respond to afatinib. J. Thorac. Oncol. 12, 446–457 (2017).
Pahuja, K. B. et al. Actionable activating oncogenic ERBB2/HER2 transmembrane and juxtamembrane domain mutations. Cancer Cell 34, 792–806.e5 (2018).
Li, B. T. et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N. Engl. J. Med. 386, 241–251 (2022).
Goto, K. et al. Trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small-cell lung cancer: primary results from the randomized, phase II DESTINY-Lung02 trial. J. Clin. Oncol. 41, 4852–4863 (2023).
Du, Z. et al. Structure-function analysis of oncogenic EGFR kinase domain duplication reveals insights into activation and a potential approach for therapeutic targeting. Nat. Commun. 12, 1382 (2021).
Govindan, R. et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 150, 1121–1134 (2012).
Wang, X. et al. Association between smoking history and tumor mutation burden in advanced non-small cell lung cancer. Cancer Res. 81, 2566–2573 (2021).
Schrock, A. B. et al. Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J. Thorac. Oncol. 12, 932–942 (2017).
Skoulidis, F. et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 8, 822–835 (2018).
Rizvi, N. A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Calles, A. et al. Expression of PD-1 and its ligands, PD-L1 and PD-L2, in smokers and never smokers with KRAS-mutant lung cancer. J. Thorac. Oncol. 10, 1726–1735 (2015).
Tseng, J. S. et al. Characteristics and predictive value of PD-L1 status in real-world non-small cell lung cancer patients. J. Immunother. 41, 292–299 (2018).
Sabari, J. K. et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann. Oncol. 29, 2085–2091 (2018).
Otano, I., Ucero, A. C., Zugazagoitia, J. & Paz-Ares, L. At the crossroads of immunotherapy for oncogene-addicted subsets of NSCLC. Nat. Rev. Clin. Oncol. 20, 143–159 (2023).
Evans, M. et al. The clinicopathological and molecular associations of PD-L1 expression in non-small cell lung cancer: analysis of a series of 10,005 cases tested with the 22c3 assay. Pathol. Oncol. Res. 26, 79–89 (2020).
Lee, J. et al. PD-L1 expression in ROS1-rearranged non-small cell lung cancer: a study using simultaneous genotypic screening of EGFR, ALK, and ROS1. Thorac. Cancer 10, 103–110 (2019).
Negrao, M. V. et al. Oncogene-specific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer. J. Immunother. Cancer 9, e002891 (2021).
Koh, J. et al. EML4-ALK enhances programmed cell death-ligand 1 expression in pulmonary adenocarcinoma via hypoxia-inducible factor (HIF)-1α and STAT3. Oncoimmunology 5, e1108514 (2016).
Shen, J. et al. PD-L1 expression is associated with ALK positivity and STAT3 activation, but not outcome in patients with systemic anaplastic large cell lymphoma. Mod. Pathol. 33, 324–333 (2020).
Nik-Zainal, S. et al. The genome as a record of environmental exposure. Mutagenesis 30, 763–770 (2015).
Yoshida, K. et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 578, 266–272 (2020).
Kim, S. H., Hwang, W. J., Cho, J. S. & Kang, D. R. Attributable risk of lung cancer deaths due to indoor radon exposure. Ann. Occup. Environ. Med. 28, 8 (2016).
Mucci, L. A. et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA 315, 68–76 (2016).
Bosse, Y. & Amos, C. I. A decade of GWAS results in lung cancer. Cancer Epidemiol. Biomark. Prev. 27, 363–379 (2018).
Mukherjee, S. et al. Germline pathogenic variants impact clinicopathology of advanced lung cancer. Cancer Epidemiol. Biomark. Prev. 31, 1450–1459 (2022).
Stadler, Z. K. et al. Therapeutic implications of germline testing in patients with advanced cancers. J. Clin. Oncol. 39, 2698–2709 (2021).
Matakidou, A., Eisen, T. & Houlston, R. S. Systematic review of the relationship between family history and lung cancer risk. Br. J. Cancer 93, 825–833 (2005).
Gorlova, O. Y. et al. Never smokers and lung cancer risk: a case-control study of epidemiological factors. Int. J. Cancer 118, 1798–1804 (2006).
Byun, J. et al. The shared genetic architectures between lung cancer and multiple polygenic phenotypes in genome-wide association studies. Cancer Epidemiol. Biomark. Prev. 30, 1156–1164 (2021).
Amos, C. I. et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat. Genet. 40, 616–622 (2008).
Hung, R. J. et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 452, 633–637 (2008).
Thorgeirsson, T. E. et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452, 638–642 (2008).
Liu, P. et al. Familial aggregation of common sequence variants on 15q24-25.1 in lung cancer. J. Natl Cancer Inst. 100, 1326–1330 (2008).
Shi, J. et al. Genome-wide association study of lung adenocarcinoma in East Asia and comparison with a European population. Nat. Commun. 14, 3043 (2023).
Li, Y. et al. Genetic variants and risk of lung cancer in never smokers: a genome-wide association study. Lancet Oncol. 11, 321–330 (2010).
McKay, J. D. et al. Lung cancer susceptibility locus at 5p15.33. Nat. Genet. 40, 1404–1406 (2008).
Hsiung, C. A. et al. The 5p15.33 locus is associated with risk of lung adenocarcinoma in never-smoking females in Asia. PLoS Genet. 6, e1001051 (2010).
Lan, Q. et al. Genome-wide association analysis identifies new lung cancer susceptibility loci in never-smoking women in Asia. Nat. Genet. 44, 1330–1335 (2012).
Rafnar, T. et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat. Genet. 41, 221–227 (2009).
Wu, X. et al. Genome-wide association study of genetic predictors of overall survival for non-small cell lung cancer in never smokers. Cancer Res. 73, 4028–4038 (2013).
Huang, Y. T. et al. Genome-wide analysis of survival in early-stage non-small-cell lung cancer. J. Clin. Oncol. 27, 2660–2667 (2009).
Wu, X. et al. Genome-wide association study of survival in non-small cell lung cancer patients receiving platinum-based chemotherapy. J. Natl Cancer Inst. 103, 817–825 (2011).
Sato, Y. et al. Genome-wide association study on overall survival of advanced non-small cell lung cancer patients treated with carboplatin and paclitaxel. J. Thorac. Oncol. 6, 132–138 (2011).
Yoon, K. A. et al. A genome-wide association study reveals susceptibility variants for non-small cell lung cancer in the Korean population. Hum. Mol. Genet. 19, 4948–4954 (2010).
Hymowitz, N., Ploshnick, A., Laemle, L. & Brezenoff, H. Effects of repeated administration of soman on schedule-controlled behavior and brain in the rat. Neurotoxicol. Teratol. 12, 47–56 (1990).
Ahn, M. J. et al. The 18p11.22 locus is associated with never smoker non-small cell lung cancer susceptibility in Korean populations. Hum. Genet. 131, 365–372 (2012).
Miki, D. et al. Variation in TP63 is associated with lung adenocarcinoma susceptibility in Japanese and Korean populations. Nat. Genet. 42, 893–896 (2010).
Wang, Y. et al. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat. Genet. 46, 736–741 (2014).
Wang, Z. et al. Meta-analysis of genome-wide association studies identifies multiple lung cancer susceptibility loci in never-smoking Asian women. Hum. Mol. Genet. 25, 620–629 (2016).
Kim, J. H. et al. Genome-wide association study of lung cancer in Korean non-smoking women. J. Korean Med. Sci. 28, 840–847 (2013).
Hosgood, H. D. III et al. Interactions between household air pollution and GWAS-identified lung cancer susceptibility markers in the Female Lung Cancer Consortium in Asia (FLCCA). Hum. Genet. 134, 333–341 (2015).
Ji, X. et al. Protein-altering germline mutations implicate novel genes related to lung cancer development. Nat. Commun. 11, 2220 (2020).
Gaughan, E. M., Cryer, S. K., Yeap, B. Y., Jackman, D. M. & Costa, D. B. Family history of lung cancer in never smokers with non-small-cell lung cancer and its association with tumors harboring EGFR mutations. Lung Cancer 79, 193–197 (2013).
Cheng, Y. I. et al. Potential genetic modifiers for somatic EGFR mutation in lung cancer: a meta-analysis and literature review. BMC Cancer 19, 1068 (2019).
Carrot-Zhang, J. et al. Genetic ancestry contributes to somatic mutations in lung cancers from admixed Latin American populations. Cancer Discov. 11, 591–598 (2021).
Kachuri, L. et al. Pan-cancer analysis demonstrates that integrating polygenic risk scores with modifiable risk factors improves risk prediction. Nat. Commun. 11, 6084 (2020).
Hung, R. J. et al. Assessing lung cancer absolute risk trajectory based on a polygenic risk model. Cancer Res. 81, 1607–1615 (2021).
Kapoor, P. M. et al. Combined associations of a polygenic risk score and classical risk factors with breast cancer risk. J. Natl Cancer Inst. 113, 329–337 (2021).
van den Broek, J. J. et al. Personalizing breast cancer screening based on polygenic risk and family history. J. Natl Cancer Inst. 113, 434–442 (2021).
Hoadley, K. A. et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 173, 291–304.e6 (2018).
Priestley, P. et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature 575, 210–216 (2019).
Hartwig Medical Foundation. Hartwig Data Catalogue https://www.hartwigmedicalfoundation.nl/en/data/database/ (2023).
ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes. Nature 578, 82–93 (2020).
Chen, J. et al. Genomic landscape of lung adenocarcinoma in East Asians. Nat. Genet. 52, 177–186 (2020).
Tlemsani, C. et al. Whole-exome sequencing reveals germline-mutated small cell lung cancer subtype with favorable response to DNA repair-targeted therapies. Sci. Transl. Med. 13, eabc7488 (2021).
Lu, T. et al. Individuals with common diseases but with a low polygenic risk score could be prioritized for rare variant screening. Genet. Med. 23, 508–515 (2021).
Tian, R. et al. Clonal hematopoiesis and risk of incident lung cancer. J. Clin. Oncol. 41, 1423–1433 (2023).
Bell, D. W. et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat. Genet. 37, 1315–1316 (2005).
Gazdar, A. et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. J. Thorac. Oncol. 9, 456–463 (2014).
Thomas, A. et al. Concurrent molecular alterations in tumors with germ line epidermal growth factor receptor T790M mutations. Clin. Lung Cancer 14, 452–456 (2013).
Yu, H. A. et al. Germline EGFR T790M mutation found in multiple members of a familial cohort. J. Thorac. Oncol. 9, 554–558 (2014).
Oxnard, G. et al. OA 06.02 final report of the INHERIT EGFR Study — 33 unrelated kindreds carrying germline EGFR mutations. J. Thorac. Oncol. 12, S1758 (2017).
Girard, N. et al. Analysis of genetic variants in never-smokers with lung cancer facilitated by an Internet-based blood collection protocol: a preliminary report. Clin. Cancer Res. 16, 755–763 (2010).
Chen, S. et al. A genome-wide mutational constraint map quantified from variation in 76,156 human genomes. Nature 625, 92–100 (2023).
Berry, D. K. et al. Clinical cohort analysis of germline EGFR T790M demonstrates penetrance across ethnicities and races, sexes, and ages. JCO Precis. Oncol. 4, PO.19.00297 (2020).
Sovich, J. L. et al. Lung adenocarcinoma associated with germline EGFR R776H variant: a case report and review of the literature. JCO Precis. Oncol. 6, e2100559 (2022).
Guo, T. et al. Two cases of non-small cell lung cancer patients with somatic or germline EGFR R776H mutation. Lung Cancer 161, 94–97 (2021).
Hellmann, M. D. et al. Identification and functional characterization of EGFR V769M, a novel germline variant associated with multiple lung adenocarcinomas. JCO Precis. Oncol. 1, PO.16.00019 (2017).
Ikeda, K., Nomori, H., Mori, T., Sasaki, J. & Kobayashi, T. Novel germline mutation: EGFR V843I in patient with multiple lung adenocarcinomas and family members with lung cancer. Ann. Thorac. Surg. 85, 1430–1432 (2008).
Ohtsuka, K. et al. Familial lung adenocarcinoma caused by the EGFR V843I germ-line mutation. J. Clin. Oncol. 29, e191-2 (2011).
van der Leest, C. et al. Novel EGFR V834L germline mutation associated with familial lung adenocarcinoma. JCO Precis. Oncol. 2, PO.17.00266 (2018).
Lu, S. et al. EGFR and ERBB2 germline mutations in Chinese lung cancer patients and their roles in genetic susceptibility to cancer. J. Thorac. Oncol. 14, 732–736 (2019).
Lin, X. et al. Identification of the unique clinical and genetic features of Chinese lung cancer patients with EGFR germline mutations in a large-scale retrospective study. Front. Oncol. 11, 774156 (2021).
Qian, K. et al. A novel germline EGFR variant p.R831H causes predisposition to familial CDK12-mutant prostate cancer with tandem duplicator phenotype. Oncogene 39, 6871–6878 (2020).
Yamamoto, H. et al. Novel germline mutation in the transmembrane domain of HER2 in familial lung adenocarcinomas. J. Natl Cancer Inst. 106, djt338 (2014).
Yamamoto, H. et al. Therapeutic potential of afatinib for cancers with ERBB2 (HER2) transmembrane domain mutations G660D and V659E. Oncologist 23, 150–154 (2018).
Yamamoto, H., Yatabe, Y. & Toyooka, S. Inherited lung cancer syndromes targeting never smokers. Transl. Lung Cancer Res. 7, 498–504 (2018).
Tode, N. et al. Exome sequencing deciphers a germline MET mutation in familial epidermal growth factor receptor-mutant lung cancer. Cancer Sci. 108, 1263–1270 (2017).
Chen, H. Y. et al. R331W missense mutation of oncogene yap1 is a germline risk allele for lung adenocarcinoma with medical actionability. J. Clin. Oncol. 33, 2303–2310 (2015).
Chaib, I. et al. Co-activation of STAT3 and YES-associated protein 1 (YAP1) pathway in EGFR-mutant NSCLC. J. Natl Cancer Inst. 109, djx014 (2017).
Kurppa, K. J. et al. Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Cancer Cell 37, 104–122.e12 (2020).
Furuya, M. et al. Pulmonary neoplasms in patients with Birt-Hogg-Dube syndrome: histopathological features and genetic and somatic events. PLoS ONE 11, e0151476 (2016).
Jia, Y. et al. Successful treatment of a patient with Li-Fraumeni syndrome and metastatic lung adenocarcinoma harboring synchronous EGFR L858R and ERBB2 extracellular domain S310F mutations with the pan-HER inhibitor afatinib. Cancer Biol. Ther. 15, 970–974 (2014).
Michalarea, V. et al. EGFR-mutated lung cancer in Li-Fraumeni syndrome. Lung Cancer 85, 485–487 (2014).
Mezquita, L. et al. High prevalence of somatic oncogenic driver alterations in patients with NSCLC and Li-fraumeni syndrome. J. Thorac. Oncol. 15, 1232–1239 (2020).
Kerrigan, K. et al. Lung cancer in Li-fraumeni syndrome. JCO Precis. Oncol. 5, PO.20.00468 (2021).
Boespflug, A. et al. Primary lung adenocarcinoma occurring in a PTEN related syndrome (Cowden’s disease): routine EGFR sequencing also highlights two rare somatic mutations S768I and V769L. Lung Cancer 79, 318–320 (2013).
Kleinerman, R. A. et al. Hereditary retinoblastoma and risk of lung cancer. J. Natl Cancer Inst. 92, 2037–2039 (2000).
Takemiya, M., Shiraishi, S., Teramoto, T. & Miki, Y. Bloom’s syndrome with porokeratosis of Mibelli and multiple cancers of the skin, lung and colon. Clin. Genet. 31, 35–44 (1987).
Yamanaka, A., Hirai, T., Ohtake, Y. & Kitagawa, M. Lung cancer associated with Werner’s syndrome: a case report and review of the literature. Jpn J. Clin. Oncol. 27, 415–418 (1997).
Zeeb, H., Shannoun, F. & World Health Organization. WHO Handbook on Indoor Radon: A Public Health Perspective (WHO, 2009).
National Research Council (US) Committee on Health Risks of Exposure to Radon (BEIR VI). Health Effects of Exposure to Radon: BEIR VI (National Academies Press, 1999).
Lubin, J. H. et al. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J. Natl Cancer Inst. 87, 817–827 (1995).
National Research Council (US) Committee on the Biological Effects of Ionizing Radiations. Health Risks of Radon and Other Internally Deposited Alpha-Emitters: Beir IV (National Academies Press, 1988).
Lubin, J. H. & Boice, J. D. Jr Lung cancer risk from residential radon: meta-analysis of eight epidemiologic studies. J. Natl Cancer Inst. 89, 49–57 (1997).
Lubin, J. H. et al. Estimating lung cancer mortality from residential radon using data for low exposures of miners. Radiat. Res. 147, 126–134 (1997).
Krewski, D. et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J. Toxicol. Environ. Health A 69, 533–597 (2006).
Zhang, Z. L. et al. Residential radon and lung cancer risk: an updated meta-analysis of case-control studies. Asian Pac. J. Cancer Prev. 13, 2459–2465 (2012).
Darby, S. et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 330, 223 (2005).
Krewski, D. et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 16, 137–145 (2005).
Lubin, J. H. et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int. J. Cancer 109, 132–137 (2004).
Garzillo, C., Pugliese, M., Loffredo, F. & Quarto, M. Indoor radon exposure and lung cancer risk: a meta-analysis of case-control studies. Transl. Cancer Res. 6, S934–S943 (2017).
Turner, M. C. et al. Radon and lung cancer in the American Cancer Society cohort. Cancer Epidemiol. Biomark. Prev. 20, 438–448 (2011).
Ngoc, L. T. N., Park, D. & Lee, Y. C. Human health impacts of residential radon exposure: updated systematic review and meta-analysis of case-control studies. Int. J. Environ. Res. Public Health 20, 97 (2022).
United States Environmental Protection Agency. A Citizen’s Guide to Radon: The Guide to Protecting Yourself and Your Family from Radon https://www.epa.gov/sites/default/files/2016-12/documents/2016_a_citizens_guide_to_radon.pdf (2012).
United States Environmental Protection Agency (EPA). The National Radon Action Plan – A Strategy for Saving Lives. epa.gov, https://www.epa.gov/radon/national-radon-action-plan-strategy-saving-lives (2023).
Lee, M. E., Lichtenstein, E., Andrews, J. A., Glasgow, R. E. & Hampson, S. E. Radon-smoking synergy: a population-based behavioral risk reduction approach. Prev. Med. 29, 222–227 (1999).
United States Environmental Protection Agency (EPA). Assessment of Risks from Radon in Homes (EPA Report No. 402-R-03-003) (Office of Radiation and Indoor Air, 2003).
Roscoe, R. J., Steenland, K., Halperin, W. E., Beaumont, J. J. & Waxweiler, R. J. Lung cancer mortality among nonsmoking uranium miners exposed to radon daughters. JAMA 262, 629–633 (1989).
Li, C. et al. Residential radon and histological types of lung cancer: a meta-analysis of case-control studies. Int. J. Environ. Res. Public Health 17, 1457 (2020).
Yao, S. X. et al. Exposure to radon progeny, tobacco use and lung cancer in a case-control study in southern China. Radiat. Res. 138, 326–336 (1994).
Rodriguez-Martinez, A., Torres-Duran, M., Barros-Dios, J. M. & Ruano-Ravina, A. Residential radon and small cell lung cancer. A systematic review. Cancer Lett. 426, 57–62 (2018).
Cheng, E. S. et al. Systematic review and meta-analysis of residential radon and lung cancer in never-smokers. Eur. Respir. Rev. 30, 200230 (2021).
Bennett, W. P. et al. Environmental tobacco smoke, genetic susceptibility, and risk of lung cancer in never-smoking women. J. Natl Cancer Inst. 91, 2009–2014 (1999).
Kiyohara, C. et al. Risk modification by CYP1A1 and GSTM1 polymorphisms in the association of environmental tobacco smoke and lung cancer: a case-control study in Japanese nonsmoking women. Int. J. Cancer 107, 139–144 (2003).
Gorlova, O. Y. et al. DNA repair capacity and lung cancer risk in never smokers. Cancer Epidemiol. Biomark. Prev. 17, 1322–1328 (2008).
Spitz, M. R. et al. Variants in inflammation genes are implicated in risk of lung cancer in never smokers exposed to second-hand smoke. Cancer Discov. 1, 420–429 (2011).
Hackshaw, A. K., Law, M. R. & Wald, N. J. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ 315, 980–988 (1997).
Lee, C. H. et al. Lifetime environmental exposure to tobacco smoke and primary lung cancer of non-smoking Taiwanese women. Int. J. Epidemiol. 29, 224–231 (2000).
Zhong, L., Goldberg, M. S., Parent, M. E. & Hanley, J. A. Exposure to environmental tobacco smoke and the risk of lung cancer: a meta-analysis. Lung Cancer 27, 3–18 (2000).
Johnson, K. C., Hu, J., Mao, Y. & Canadian Cancer Registries Epidemiology Research Group. Lifetime residential and workplace exposure to environmental tobacco smoke and lung cancer in never-smoking women, Canada 1994-97. Int. J. Cancer 93, 902–906 (2001).
Wang, A. et al. Active and passive smoking in relation to lung cancer incidence in the Women’s Health Initiative prospective cohort study. J. Clin. Oncol. 31, 1504 (2013). 1504.
Couraud, S. et al. No impact of passive smoke on the somatic profile of lung cancers in never-smokers. Eur. Respir. J. 45, 1415–1425 (2015).
International Agency for Research on Cancer, World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Tobacco Smoke and Involuntary Smoking Vol. 83 (IARC, WHO, 2004).
Office of the Surgeon General, Office on Smoking and Health (US). The Health Consequences of Smoking: A Report of the Surgeon General (Centers for Disease Control and Prevention, 2004).
Olsson, A. C. et al. Exposure to diesel motor exhaust and lung cancer risk in a pooled analysis from case-control studies in Europe and Canada. Am. J. Respir. Crit. Care Med. 183, 941–948 (2011).
Silverman, D. T. et al. The diesel exhaust in miners study: a nested case-control study of lung cancer and diesel exhaust. J. Natl Cancer Inst. 104, 855–868 (2012).
Raaschou-Nielsen, O. et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 14, 813–822 (2013).
GBD 2019 Respiratory Tract Cancers Collaborators Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir. Med. 9, 1030–1049 (2021).
Huang, Y. et al. Air pollution, genetic factors, and the risk of lung cancer: a prospective study in the UK biobank. Am. J. Respir. Crit. Care Med. 204, 817–825 (2021).
Krewski, D. et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res. Rep. Health Eff. Inst. 140, 5–114 (2009).
Turner, M. C. et al. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am. J. Respir. Crit. Care Med. 184, 1374–1381 (2011).
International Agency for Research on Cancer, World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Outdoor Air Pollution Vol. 109 (IARC, WHO, 2016).
Brook, R. D. et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378 (2010).
Cohen, A. J. et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389, 1907–1918 (2017).
Turner, M. C. et al. Interactions between cigarette smoking and fine particulate matter in the Risk of Lung Cancer Mortality in Cancer Prevention Study II. Am. J. Epidemiol. 180, 1145–1149 (2014).
Myers, R. et al. High-ambient air pollution exposure among never smokers versus ever smokers with lung cancer. J. Thorac. Oncol. 16, 1850–1858 (2021).
Hoffmann, B. et al. WHO Air Quality Guidelines 2021 — aiming for healthier air for all: a joint statement by medical, public health, scientific societies and patient representative organisations. Int. J. Public Health 66, 1604465 (2021).
Shi, L. et al. Low-concentration PM2.5 and mortality: estimating acute and chronic effects in a population-based study. Environ. Health Perspect. 124, 46–52 (2016).
United States Environmental Protection Agency. National Ambient Air Quality Standards (NAAQS) for PM https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm (2023).
Di, Q. et al. Daily and Annual PM2.5 Concentrations for the Contiguous United States, 1-km Grids, v1 (2000–2016) (NASA Socioeconomic Data and Applications Center, 2021).
Inness, A. et al. The CAMS reanalysis of atmospheric composition. Atmos. Chem. Phys. 19, 3515–3556 (2019).
Christiani, D. C. Ambient air pollution and lung cancer: nature and nurture. Am. J. Respir. Crit. Care Med. 204, 752–753 (2021).
Hill, W. et al. Lung adenocarcinoma promotion by air pollutants. Nature 616, 159–167 (2023).
Jbaily, A. et al. Air pollution exposure disparities across US population and income groups. Nature 601, 228–233 (2022).
Loomis, D., Guha, N., Hall, A. L. & Straif, K. Identifying occupational carcinogens: an update from the IARC Monographs. Occup. Environ. Med. 75, 593–603 (2018).
Ge, C. et al. Respirable crystalline silica exposure, smoking, and lung cancer subtype risks. a pooled analysis of case-control studies. Am. J. Respir. Crit. Care Med. 202, 412–421 (2020).
Ge, C. et al. Diesel engine exhaust exposure, smoking, and lung cancer subtype risks. a pooled exposure-response analysis of 14 case-control studies. Am. J. Respir. Crit. Care Med. 202, 402–411 (2020).
Honaryar, M. K. et al. Welding fumes and lung cancer: a meta-analysis of case-control and cohort studies. Occup. Environ. Med. 76, 422–431 (2019).
Banks, D. E. et al. American College of Chest Physicians consensus statement on the respiratory health effects of asbestos. Results of a Delphi study. Chest 135, 1619–1627 (2009).
Markowitz, S. B., Levin, S. M., Miller, A. & Morabia, A. Asbestos, asbestosis, smoking, and lung cancer. New findings from the North American insulator cohort. Am. J. Respir. Crit. Care Med. 188, 90–96 (2013).
Bridda, A., Padoan, I., Mencarelli, R. & Frego, M. Peritoneal mesothelioma: a review. MedGenMed 9, 32 (2007).
Mazurek, J. M., Syamlal, G., Wood, J. M., Hendricks, S. A. & Weston, A. Malignant mesothelioma mortality — United States, 1999-2015. MMWR Morb. Mortal. Wkly Rep. 66, 214–218 (2017).
Selikoff, I. J., Hammond, E. C. & Churg, J. Asbestos exposure, smoking, and neoplasia. JAMA 204, 106–112 (1968).
Lee, P. N. Relation between exposure to asbestos and smoking jointly and the risk of lung cancer. Occup. Environ. Med. 58, 145–153 (2001).
Liddell, F. D. The interaction of asbestos and smoking in lung cancer. Ann. Occup. Hyg. 45, 341–356 (2001).
Ngamwong, Y. et al. Additive synergism between asbestos and smoking in lung cancer risk: a systematic review and meta-analysis. PLoS ONE 10, e0135798 (2015).
Nelson, H. H. et al. k-ras mutation and occupational asbestos exposure in lung adenocarcinoma: asbestos-related cancer without asbestosis. Cancer Res. 59, 4570–4573 (1999).
Kwak, K., Kang, D. & Paek, D. Environmental exposure to asbestos and the risk of lung cancer: a systematic review and meta-analysis. Occup. Environ. Med. 79, 207–214 (2022).
Murray, R. P., Connett, J. E. & Zapawa, L. M. Does nicotine replacement therapy cause cancer? Evidence from the Lung Health Study. Nicotine Tob. Res. 11, 1076–1082 (2009).
Lee, P. N. & Fariss, M. W. A systematic review of possible serious adverse health effects of nicotine replacement therapy. Arch. Toxicol. 91, 1565–1594 (2017).
Sheikh, M., Mukeriya, A., Shangina, O., Brennan, P. & Zaridze, D. Postdiagnosis smoking cessation and reduced risk for lung cancer progression and mortality: a prospective cohort study. Ann. Intern. Med. 174, 1232–1239 (2021).
Fares, A. F. et al. Association between duration of smoking abstinence before non-small-cell lung cancer diagnosis and survival: a retrospective, pooled analysis of cohort studies. Lancet Public Health 8, e691–e700 (2023).
Caini, S. et al. Quitting smoking at or around diagnosis improves the overall survival of lung cancer patients: a systematic review and meta-analysis. J. Thorac. Oncol. 17, 623–636 (2022).
Godtfredsen, N. S., Prescott, E. & Osler, M. Effect of smoking reduction on lung cancer risk. JAMA 294, 1505–1510 (2005).
Christiani, D. C. Vaping-induced acute lung injury. N. Engl. J. Med. 382, 960–962 (2020).
Kosmider, L. et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob. Res. 16, 1319–1326 (2014).
Mulder, H. A. et al. The effect of electronic cigarette user modifications and e-liquid adulteration on the particle size profile of an aerosolized product. Sci. Rep. 9, 10221 (2019).
Lalo, H., Leclerc, L., Sorin, J. & Pourchez, J. Aerosol droplet-size distribution and airborne nicotine portioning in particle and gas phases emitted by electronic cigarettes. Sci. Rep. 10, 21707 (2020).
Goniewicz, M. L. et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control. 23, 133–139 (2014).
Jensen, R. P., Luo, W., Pankow, J. F., Strongin, R. M. & Peyton, D. H. Hidden formaldehyde in e-cigarette aerosols. N. Engl. J. Med. 372, 392–394 (2015).
Laino, T. et al. Mechanisms of propylene glycol and triacetin pyrolysis. J. Phys. Chem. A 116, 4602–4609 (2012).
Ingebrethsen, B. J., Cole, S. K. & Alderman, S. L. Electronic cigarette aerosol particle size distribution measurements. Inhal. Toxicol. 24, 976–984 (2012).
Eversole, A. et al. E-cigarette solvent ratio and device power influence ambient air particulate matter. Tob. Regul. Sci. 7, 177–183 (2021).
International Agency for Research on Cancer, World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Household Use of Solid Fuels and High-temperature Frying Vol. 95 (IARC, WHO, 2010).
Jia, P. L. et al. The risk of lung cancer among cooking adults: a meta-analysis of 23 observational studies. J. Cancer Res. Clin. Oncol. 144, 229–240 (2018).
Xue, Y., Jiang, Y., Jin, S. & Li, Y. Association between cooking oil fume exposure and lung cancer among Chinese nonsmoking women: a meta-analysis. Onco Targets Ther. 9, 2987–2992 (2016).
Yu, I. T., Chiu, Y. L., Au, J. S., Wong, T. W. & Tang, J. L. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 66, 4961–4967 (2006).
Barone-Adesi, F. et al. Risk of lung cancer associated with domestic use of coal in Xuanwei, China: retrospective cohort study. BMJ 345, e5414 (2012).
Kurmi, O. P., Arya, P. H., Lam, K. B., Sorahan, T. & Ayres, J. G. Lung cancer risk and solid fuel smoke exposure: a systematic review and meta-analysis. Eur. Respir. J. 40, 1228–1237 (2012).
Naeher, L. P. et al. Woodsmoke health effects: a review. Inhal. Toxicol. 19, 67–106 (2007).
Mehta, S. S. et al. Indoor wood-burning from stoves and fireplaces and incident lung cancer among Sister Study participants. Environ. Int. 178, 108128 (2023).
Pagadala, M. et al. Germline modifiers of the tumor immune microenvironment implicate drivers of cancer risk and immunotherapy response. Nat. Commun. 14, 2744 (2023).
Luo, J. et al. Immunotherapy-mediated thyroid dysfunction: genetic risk and impact on outcomes with PD-1 blockade in non-small cell lung cancer. Clin. Cancer Res. 27, 5131–5140 (2021).
Groha, S. et al. Germline variants associated with toxicity to immune checkpoint blockade. Nat. Med. 28, 2584–2591 (2022).
National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 5.2023), https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed 4 August 2023).
M. D. Anderson Cancer Center & National Cancer Institute. Local consolidative therapy and brigatinib in treating patients with stage IV or recurrent non-small cell lung cancer. ClinicalTrials.gov, https://clinicaltrials.gov/study/NCT03707938 (2018).
M. D. Anderson Cancer Center, National Cancer Institute & National Comprehensive Cancer Network. Osimertinib, surgery, and radiation therapy in treating patients with stage IIIB or IV non-small cell lung cancer with EGFR mutations, NORTHSTAR study. ClinicalTrials.gov, https://clinicaltrials.gov/study/NCT03410043 (2018).
Elamin, Y. Y. et al. Local consolidation therapy (LCT) after first line tyrosine kinase inhibitor (TKI) for patients with EGFR mutant metastatic non-small-cell lung cancer (NSCLC). Clin. Lung Cancer 20, 43–47 (2019).
Zeng, Y. et al. The value of local consolidative therapy in osimertinib-treated non-small cell lung cancer with oligo-residual disease. Radiat. Oncol. 15, 207 (2020).
Planchard, D. et al. Osimertinib with or without chemotherapy in EGFR-mutated advanced NSCLC. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2306434 (2023).
Solomon, B. J. et al. ALINA: a phase III study of alectinib versus chemotherapy as adjuvant therapy in patients with stage IB–IIIA anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer (NSCLC). J. Clin. Oncol. 37, TPS8569 (2019).
Herbst, R. S. et al. Adjuvant osimertinib for resected EGFR-mutated stage IB-IIIA non-small-cell lung cancer: updated results from the phase III randomized ADAURA trial. J. Clin. Oncol. 41, 1830–1840 (2023).
Urisman, A. et al. Phase II trial of neoadjuvant osimertinib for surgically resectable EGFR-mutated non-small cell lung cancer. J. Clin. Oncol. 41, 8508–8508 (2023).
Solomon, B. J. et al. LBA2 ALINA: efficacy and safety of adjuvant alectinib versus chemotherapy in patients with early-stage ALK+ non-small cell lung cancer (NSCLC). Ann. Oncol. 34, S1295–S1296 (2023).
Tsuboi, M. et al. Overall survival with osimertinib in resected EGFR-mutated NSCLC. N. Engl. J. Med. 389, 137–147 (2023).
National Lung Screening Trial Research Teamet al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 365, 395–409 (2011).
de Koning, H. J. et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 382, 503–513 (2020).
Khuder, S. A. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer 31, 139–148 (2001).
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020).
Ernst, S. M. et al. Tobacco smoking-related mutational signatures in classifying smoking-associated and nonsmoking-associated NSCLC. J. Thorac. Oncol. 18, 487–498 (2023).
Lindsay, C. R. et al. Abstract 6463: persistence of smoking mutational signatures in the non-small cell lung cancer genome. Cancer Res. 83, 6463–6463 (2023).
Wang, Y., Broderick, P., Matakidou, A., Eisen, T. & Houlston, R. S. Role of 5p15.33 (TERT-CLPTM1L), 6p21.33 and 15q25.1 (CHRNA5-CHRNA3) variation and lung cancer risk in never-smokers. Carcinogenesis 31, 234–238 (2010).
Hung, R. J. et al. Lung cancer risk in never-smokers of European descent is associated with genetic variation in the 5(p)15.33 TERT-CLPTM1Ll region. J. Thorac. Oncol. 14, 1360–1369 (2019).
Hosgood, H. D. III et al. Genetic variant in TP63 on locus 3q28 is associated with risk of lung adenocarcinoma among never-smoking females in Asia. Hum. Genet. 131, 1197–1203 (2012).
Shiraishi, K. et al. A genome-wide association study identifies two new susceptibility loci for lung adenocarcinoma in the Japanese population. Nat. Genet. 44, 900–903 (2012).
Yngveson, A. et al. p53 Mutations in lung cancer associated with residential radon exposure. Cancer Epidemiol. Biomark. Prev. 8, 433–438 (1999).
Peres, J. No clear link between passive smoking and lung cancer. J. Natl Cancer Inst. 105, 1844–1846 (2013).
Hammond, E. C., Selikoff, I. J. & Seidman, H. Asbestos exposure, cigarette smoking and death rates. Ann. N. Y. Acad. Sci. 330, 473–490 (1979).
Sichletidis, L. et al. Mortality from occupational exposure to relatively pure chrysotile: a 39-year study. Respiration 78, 63–68 (2009).
Shapiro, M. Z. et al. Cancer in general responders participating in World Trade Center Health Programs, 2003-2013. JNCI Cancer Spectr. 4, pkz090 (2020).
Ward, A. M., Yaman, R. & Ebbert, J. O. Electronic nicotine delivery system design and aerosol toxicants: a systematic review. PLoS ONE 15, e0234189 (2020).
Goniewicz, M. L. et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw. Open 1, e185937 (2018).
Shahab, L. et al. Nicotine, carcinogen, and toxin exposure in long-term E-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann. Intern. Med. 166, 390–400 (2017).
Barcenas, C. H. et al. Wood dust exposure and the association with lung cancer risk. Am. J. Ind. Med. 47, 349–357 (2005).
Tanvetyanon, T. & Bepler, G. Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: a meta-analysis and evaluation of national brands. Cancer 113, 150–157 (2008).
Omenn, G. S. et al. Chemoprevention of lung cancer: the beta-Carotene and Retinol Efficacy Trial (CARET) in high-risk smokers and asbestos-exposed workers. IARC Sci. Publ. 136, 67–85 (1996).
Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 330, 1029–1035 (1994).
Brasky, T. M., White, E. & Chen, C. L. Long-term, supplemental, one-carbon metabolism-related vitamin B use in relation to lung cancer risk in the vitamins and lifestyle (VITAL) cohort. J. Clin. Oncol. 35, 3440–3448 (2017).
Hennekens, C. H. et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 334, 1145–1149 (1996).
Chlebowski, R. T. et al. Lung cancer among postmenopausal women treated with estrogen alone in the women’s health initiative randomized trial. J. Natl Cancer Inst. 102, 1413–1421 (2010).
Chlebowski, R. T. et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet 374, 1243–1251 (2009).
Heiss, G. et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA 299, 1036–1045 (2008).
Slatore, C. G., Chien, J. W., Au, D. H., Satia, J. A. & White, E. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J. Clin. Oncol. 28, 1540–1546 (2010).
Pesatori, A. C. et al. Hormone use and risk for lung cancer: a pooled analysis from the International Lung Cancer Consortium (ILCCO). Br. J. Cancer 109, 1954–1964 (2013).
Seow, A., Koh, W. P., Wang, R., Lee, H. P. & Yu, M. C. Reproductive variables, soy intake, and lung cancer risk among nonsmoking women in the Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 18, 821–827 (2009).
Weiss, J. M. et al. Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. Am. J. Epidemiol. 168, 1319–1325 (2008).
Ganti, A. K., Sahmoun, A. E., Panwalkar, A. W., Tendulkar, K. K. & Potti, A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J. Clin. Oncol. 24, 59–63 (2006).
Liu, Y., Inoue, M., Sobue, T. & Tsugane, S. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: a large-scale population-based cohort study. Int. J. Cancer 117, 662–666 (2005).
Hopkins, R. J. et al. Reduced expiratory flow rate among heavy smokers increases lung cancer risk. results from the National Lung Screening Trial — American College of Radiology Imaging Network Cohort. Ann. Am. Thorac. Soc. 14, 392–402 (2017).
Denholm, R. et al. Is previous respiratory disease a risk factor for lung cancer? Am. J. Respir. Crit. Care Med. 190, 549–559 (2014).
Brenner, D. R. et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am. J. Epidemiol. 176, 573–585 (2012).
Brenner, D. R., McLaughlin, J. R. & Hung, R. J. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS ONE 6, e17479 (2011).
Shiels, M. S., Albanes, D., Virtamo, J. & Engels, E. A. Increased risk of lung cancer in men with tuberculosis in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol. Biomark. Prev. 20, 672–678 (2011).
Yu, Y. H. et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J. Thorac. Oncol. 6, 32–37 (2011).
Jacob, S. et al. Lung cancer survival in patients with autoimmune disease. JAMA Netw. Open 3, e2029917 (2020).
Liu, X. et al. Clinicopathological features of lung cancer in patients with rheumatoid arthritis. J. Thorac. Dis. 10, 3965–3972 (2018).
Naccache, J. M. et al. Lung cancer and interstitial lung disease: a literature review. J. Thorac. Dis. 10, 3829–3844 (2018).
Onishi, A., Sugiyama, D., Kumagai, S. & Morinobu, A. Cancer incidence in systemic sclerosis: meta-analysis of population-based cohort studies. Arthritis Rheum. 65, 1913–1921 (2013).
Siemes, C. et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J. Clin. Oncol. 24, 5216–5222 (2006).
Sheikh, M. et al. Opium use and subsequent incidence of cancer: results from the Golestan Cohort Study. Lancet Glob. Health 8, e649–e660 (2020).
IARC Monographs Vol 126 Group. Carcinogenicity of opium consumption. Lancet Oncol. 21, 1407–1408 (2020).
Rahimi-Movaghar, A. et al. Pharmacological therapies for management of opium withdrawal. Cochrane Database Syst. Rev. 6, CD007522 (2018).
Callaghan, R. C., Allebeck, P. & Sidorchuk, A. Marijuana use and risk of lung cancer: a 40-year cohort study. Cancer Causes Control. 24, 1811–1820 (2013).
Tashkin, D. P. Effects of marijuana smoking on the lung. Ann. Am. Thorac. Soc. 10, 239–247 (2013).
Sarafian, T. A., Magallanes, J. A., Shau, H., Tashkin, D. & Roth, M. D. Oxidative stress produced by marijuana smoke. An adverse effect enhanced by cannabinoids. Am. J. Respir. Cell Mol. Biol. 20, 1286–1293 (1999).
Barsky, S. H., Roth, M. D., Kleerup, E. C., Simmons, M. & Tashkin, D. P. Histopathologic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. J. Natl Cancer Inst. 90, 1198–1205 (1998).
Lorigan, P., Radford, J., Howell, A. & Thatcher, N. Lung cancer after treatment for Hodgkin’s lymphoma: a systematic review. Lancet Oncol. 6, 773–779 (2005).
Huang, Y. J. et al. Radiation therapy for invasive breast cancer increases the risk of second primary lung cancer: a nationwide population-based cohort analysis. J. Thorac. Oncol. 12, 782–790 (2017).
Cornelius, M. E., Loretan, C. G., Wang, T. W., Jamal, A. & Homa, D. M. Tobacco product use among adults — United States, 2020. MMWR Morb. Mortal. Wkly Rep. 71, 397–405 (2022).
Cornelius, M. E., Wang, T. W., Jamal, A., Loretan, C. G. & Neff, L. J. Tobacco product use among adults — United States, 2019. MMWR Morb. Mortal. Wkly Rep. 69, 1736–1742 (2020).
Suehnholz, S. P. et al. Quantifying the expanding landscape of clinical actionability for patients with cancer. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-23-0467 (2023).
Chakravarty, D. et al. OncoKB: a precision oncology knowledge base. JCO Precis. Oncol. 2017, PO.17.00011 (2017).
Chang, M. T. et al. Accelerating discovery of functional mutant alleles in cancer. Cancer Discov. 8, 174–183 (2018).
Chang, M. T. et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat. Biotechnol. 34, 155–163 (2016).
Acknowledgements
The authors gratefully acknowledge M. Makarem, R. Collins, I. Odintsov and B. Johnson (at the Dana-Farber Cancer Institute) for their careful review of the manuscript and/or thoughtful discussions as well as Z. Wei and A. Tramontano (at the Dana-Farber Cancer Institute) and A. Shtein and Y. Wei (at the Harvard School of Public Health) for their assistance with data gathering and visualization.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and contributed substantially to the discussion of content. J.L. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P.A.J. declares consultancy roles for Abbvie, Accutar Biotech, Allorion Therapeutics, AstraZeneca, Bayer, Biocartis, Boehringer Ingelheim, Chugai Pharmaceuticals, Daiichi Sankyo, DualityBio, Eisai, Eli Lilly, Frontier Medicines, Hongyun Biotechnology, Merus, Mirati Therapeutics, Monte Rosa, Novartis, Nuvalent, Pfizer, Roche/Genentech, Sanofi, Scorpion Therapeutics, SFJ Pharmaceuticals, Silicon Therapeutics, Syndax, Takeda Oncology, Transcenta and Voronoi; stock ownership in Gatekeeper Pharmaceuticals; research funding from AstraZenenca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, PUMA Biotechnology, Revolution Medicines and Takeda Oncology; and post-marketing royalties from Dana-Farber Cancer Institute-owned intellectual property relating to EGFR mutations that has been licensed to Lab Corp. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Cancer Hotspots: https://www.cancerhotspots.org/#/home
ClinicalTrials.gov: https://clinicaltrials.gov/
GLOBOCAN: https://gco.iarc.fr/today/home
National Comprehensive Cancer Network (NCCN) Guidelines for NSCLC: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450
OncoKB: https://www.oncokb.org/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
LoPiccolo, J., Gusev, A., Christiani, D.C. et al. Lung cancer in patients who have never smoked — an emerging disease. Nat Rev Clin Oncol 21, 121–146 (2024). https://doi.org/10.1038/s41571-023-00844-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00844-0