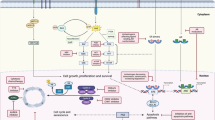
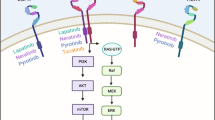
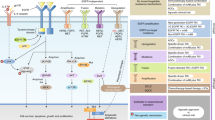
Abstract
Laboratory studies that led to the development of epidermal growth factor receptor (EGFR) inhibitors indicated that such inhibitors would be effective when given to patients with tumours that are driven by activated EGFR. However, initial clinical studies have shown modest responses to EGFR inhibitors when used alone, and it has not yet been possible to clearly identify which tumours will respond to this therapy. As a result, EGFR inhibitors are now used in combination with radiation therapy, chemotherapy and, more recently, with concurrent radiochemotherapy. In general, these clinical trials have been designed without much preclinical data. What do we need to know to make these combinations successful in the clinic?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Steel, G. G. & Peckham, M. J. Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int. J. Radiat. Onc. Biol. Phys. 5, 85–91 (1979).
Chou, T.-C. & Talalay, P. Quantitative analysis of dose-effective relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances Enzyme Res. 22, 27–55 (1984).
Tannock, I. F. Treatment of cancer with radiation and drugs. J. Clin. Oncol. 14, 3156–3174 (1996).
Pignon, J. P. et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 355, 949–955 (2000).
Dillman, R. O. et al. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J. Natl Cancer Inst. 88, 1210–1215 (1996).
Vokes, E. E. Optimal therapy for unresectable stage III non-small-cell lung cancer. J. Clin. Oncol. 23, 5853–5855 (2005).
O'Brien, S. G. et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 348, 994–1004 (2003).
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumours. N. Engl. J. Med. 347, 472–480 (2002).
Kantarjian, H. et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med. 346, 645–652 (2002).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006).
Gullick, W. J. et al. The structure and function of the epidermal growth factor receptor studied by using antisynthetic peptide antibodies. Proc. R. Soc. Lond. B 226, 127–134 (1985).
Mendelsohn, J. & Baselga, J. The EGF receptor family as targets for cancer therapy. Oncogene 19, 6550–6565 (2000).
Ward, W. H. et al. Epidermal growth factor receptor tyrosine kinase. Investigation of catalytic mechanism, structure-based searching and discovery of a potent inhibitor. Biochem. Pharmacol. 48, 659–666 (1994).
Levitzki, A. & Gazit, A. Tyrosine kinase inhibition: an approach to drug development. Science 267, 1782–1788 (1995).
Goldstein, N. I., Prewett, M., Zuklys, K., Rockwell, P. & Mendelsohn, J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumour xenograft model. Clin. Cancer Res. 1, 1311–1318 (1995).
Fry, D. W. et al. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc. Natl Acad. Sci. USA 95, 12022–12027 (1998).
Ciardiello, F. et al. Antitumour effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin. Cancer Res. 6, 2053–2063 (2000).
Stamos, J., Sliwkowski, M. X. & Eigenbrot, C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 277, 46265–46272 (2002).
Mendelsohn, J. & Fan, Z. Epidermal growth factor receptor family and chemosensitization. J. Natl Cancer Inst. 89, 341–343 (1997).
Milas, L. et al. In vivo enhancement of tumour radioresponse by C225 antiepidermal growth factor receptor antibody. Clin. Cancer Res. 6, 701–708 (2000).
Harari, P. M. & Huang, S. M. Head and neck cancer as a clinical model for molecular targeting of therapy: combining EGFR blockade with radiation. Int. J. Rad. Oncol. Biol. Phys. 49, 427–433 (2001).
Herbst, R. S., Kim, E. S. & Harari, P. M. IMC-C225, an anti-epidermal growth factor receptor monoclonal antibody, for treatment of head and neck cancer. Expert Opin. Biol. Ther. 1, 719–732 (2001).
Solomon, B. et al. EGFR blockade with ZD1839 ('Iressa') potentiates the antitumour effects of single and multiple fractions of ionizing radiation in human A431 squamous cell carcinoma. Epidermal growth factor receptor. Int. J. Radiat. Oncol. Biol. Phys. 55, 713–723 (2003).
Milano, G. & Magne, N. Anti-EGFR and radiotherapy. Cancer Radiother. 8, 380–382 (2004).
Nyati, M. K. et al. Radiosensitization by pan ErbB inhibitor CI-1033 in vitro and in vivo. Clin. Cancer Res. 10, 691–700 (2004).
Deutsch, E., Kaliski, A., Maggiorella, L. & Bourhis, J. New strategies to interfere with radiation response: 'biomodulation' of radiation therapy. Cancer Radiother. 9, 69–76 (2005).
Yarden, Y. & Sliwkowski, M. X. Untangling the ErbB signalling network. Nature Rev. Mol. Cell Biol. 2, 127–137 (2001).
Parra, H. S. et al. Analysis of epidermal growth factor receptor expression as a predictive factor for response to gefitinib ('Iressa', ZD1839) in non-small-cell lung cancer. Br. J. Cancer 91, 208–212 (2004).
Han, S. W. et al. Epidermal growth factor receptor (EGFR) downstream molecules as response predictive markers for gefitinib (Iressa, ZD1839) in chemotherapy-resistant non-small cell lung cancer. Int. J. Cancer 113, 109–115 (2005).
Cappuzzo, F. et al. Gefitinib in pretreated non-small-cell lung cancer (NSCLC): analysis of efficacy and correlation with HER2 and epidermal growth factor receptor expression in locally advanced or metastatic NSCLC. J. Clin. Oncol. 21, 2658–2663 (2003).
Cappuzzo, F. et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J. Natl Cancer Inst. 97, 643–655 (2005).
Hirsch, F. R. et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J. Clin. Oncol. 23, 6838–6845 (2005).
Takano, T. et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J. Clin. Oncol. 23, 6829–6837 (2005).
Johnson, B. E. & Janne, P. A. Selecting patients for epidermal growth factor receptor inhibitor treatment: a FISH story or a tale of mutations? J. Clin. Oncol. 23, 6813–6816 (2005).
Garcia de Palazzo, I. E. et al. Expression of mutated epidermal growth factor receptor by non-small cell lung carcinomas. Cancer Res. 53, 3217–3220 (1993).
Moscatello, D. K. et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumours. Cancer Res. 55, 5536–5539 (1995).
Mellinghoff, I. K. et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024 (2005).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Janne, P. A., Engelman, J. A. & Johnson, B. E. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumour biology. J. Clin. Oncol. 23, 3227–3234 (2005).
Dowell, J. E. Epidermal growth factor receptor mutations in non-small cell lung cancer: a basic science discovery with immediate clinical impact. Am. J. Med. Sci. 331, 139–149 (2006).
Kwok, T. T. & Sutherland, R. M. Enhancement of sensitivity of human squamous carcinoma cells to radiation by epidermal growth factor. J. Natl Cancer Inst. 81, 1020–1024 (1989).
Bonner, J. A., Maihle, N. J., Folven, B. R., Christianson, T. J. & Spain, K. The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. Int. J. Radiat. Oncol. Biol. Phys. 29, 243–247 (1994).
Balaban, N. et al. The effect of ionizing radiation on signal transduction: Antibodies to EGF receptor sensitize A431 cells to radiation. Biochim. Biophys. Acta 1314, 147–156 (1996).
Sheridan, M. T., O'Dwyer, T., Seymour, C. B. & Mothersill, C. E. Potential indicators of radiosensitivity in squamous cell carcinoma of the head and neck. Radiat. Oncol. Investig. 5, 180–186 (1997).
Akimoto, T. et al. Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin. Cancer Res. 5, 2884–2890 (1999).
Milas, L., Fan, Z., Andratschke, N. H. & Ang, K. K. Epidermal growth factor receptor and tumour response to radiation: in vivo preclinical studies. Int. J. Rad. Oncol. Biol. Phys. 58, 966–971 (2004).
Ang, K. K. et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 62, 7350–7356 (2002).
Peng, D. et al. Anti-epidermal growth factor receptor monoclonal antibody 225 upregulates p27KIP1 and induces G1 arrest in prostatic cancer cell line DU145. Cancer Res. 56, 3666–3669 (1996).
Di Gennaro, E. et al. Critical role of both p27KIP1 and p21CIP1/WAF1 in the antiproliferative effect of ZD1839 ('Iressa'), an epidermal growth factor receptor tyrosine kinase inhibitor, in head and neck squamous carcinoma cells. J. Cell Physiol. 195, 139–150 (2003).
Schmidt-Ullrich, R. K., Valerie, K., Fogleman, P. B. & Walters, J. Radiation-induced autophosphorylation of epidermal growth factor receptor in human malignant mammary and squamous epithelial cells. Radiat. Res. 145, 81–85 (1996).
Schmidt-Ullrich, R. K. et al. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene 15, 1191–1197 (1997).
Dent, P. et al. Radiation-induced release of transforming growth factor alpha activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol. Biol. Cell 10, 2493–2506 (1999).
Contessa, J. N. et al. Ionizing radiation activates Erb-B receptor dependent Akt and p70 S6 kinase signalling in carcinoma cells. Oncogene 21, 4032–4041 (2002).
Dittmann, K. et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J. Biol. Chem. 280, 31182–31189 (2005).
Bowers, G. et al. The relative role of ErbB1–4 receptor tyrosine kinases in radiation signal transduction responses of human carcinoma cells. Oncogene 20, 1388–1397 (2001).
Hagan, M., Yacoub, A. & Dent, P. Ionizing radiation causes a dose-dependent release of transforming growth factor alpha in vitro from irradiated xenografts and during palliative treatment of hormone-refractory prostate carcinoma. Clin. Cancer Res. 10, 5724–5731 (2004).
Huang, S. M., Bock, J. M. & Harari, P. M. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 59, 1935–1940 (1999).
Rao, G. S., Murray, S. & Ethier, S. P. Radiosensitization of human breast cancer cells by a novel ErbB family receptor tyrosine kinase inhibitor. Int. J. Rad. Oncol. Biol. Phys. 48, 1519–1528 (2000).
Bonner, J. A. et al. Enhanced apoptosis with combination C225/radiation treatment serves as the impetus for clinical investigation in head and neck cancers. J. Clin. Oncol. 18, 47S–53S (2000).
Bianco, C. et al. Antitumour activity of combined treatment of human cancer cells with ionizing radiation and anti-epidermal growth factor receptor monoclonal antibody C225 plus type I protein kinase A antisense oligonucleotide. Clin. Cancer Res. 6, 4343–4350 (2000).
Shintani, S. et al. Enhancement of tumour radioresponse by combined treatment with gefitinib (Iressa, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, is accompanied by inhibition of DNA damage repair and cell growth in oral cancer. Int. J. Cancer 107, 1030–1037 (2003).
Saleh, M. N. et al. Combined modality therapy of A431 human epidermoid cancer using anti-EGFr antibody C225 and radiation. Cancer Biother. Radiopharm. 14, 451–463 (1999).
Huang, S. M. & Harari, P. M. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumour angiogenesis. Clin. Cancer Res. 6, 2166–2174 (2000).
Harari, P. M. & Huang, S. M. Modulation of molecular targets to enhance radiation. Clin. Cancer Res. 6, 323–325 (2000).
Li, J., Lin, M. L., Wiepz, G. J., Guadarrama, A. G. & Bertics, P. J. Integrin-mediated migration of murine B82L fibroblasts is dependent on the expression of an intact epidermal growth factor receptor. J. Biol. Chem. 274, 11209–11219 (1999).
Raben, D., Helfrich, B. & Bunn, P. A. Targeted therapies for non-small-cell lung cancer: biology, rationale, and preclinical results from a radiation oncology perspective. Int. J. Rad. Oncol. Biol. Phys. 59, 27–38 (2004).
Robert, F. et al. Phase I study of anti--epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J. Clin. Oncol. 19, 3234–3243 (2001).
Matar, P. et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin. Cancer Res. 10, 6487–6501 (2004).
Huang, S., Armstrong, E. A., Benavente, S., Chinnaiyan, P. & Harari, P. M. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 64, 5355–5362 (2004).
Baselga, J. et al. Antitumour effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J. Natl Cancer Inst. 85, 1327–1333 (1993).
Fan, Z., Baselga, J., Masui, H. & Mendelsohn, J. Antitumour effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 53, 4637–4642 (1993).
Sirotnak, F. M., Zakowski, M. F., Miller, V. A., Scher, H. I. & Kris, M. G. Efficacy of cytotoxic agents against human tumour xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin. Cancer Res. 6, 4885–4892 (2000).
Busse, D. et al. Reversible G(1) arrest induced by inhibition of the epidermal growth factor receptor tyrosine kinase requires up-regulation of p27 (KIP1) independent of MAPK activity. J. Biol.Chem. 275, 6987–6995 (2000).
Wu, X. et al. Involvement of p27KIP1 in G1 arrest mediated by an anti-epidermal growth factor receptor monoclonal antibody. Oncogene 12, 1397–1403 (1996).
Morelli, M. P. et al. Sequence-dependent antiproliferative effects of cytotoxic drugs and epidermal growth factor receptor inhibitors. Ann. Oncol. 16 (Suppl. 4), iv61–iv68 (2005).
Chun, P. Y. et al. Synergistic effects of gemcitabine and gefitinib in the treatment of head and neck carcinoma. Cancer Res. 66, 981–988 (2006).
Azzariti, A., Xu, J. M., Porcelli, L. & Paradiso, A. The schedule-dependent enhanced cytotoxic activity of 7-ethyl-10-hydroxy-camptothecin (SN-38) in combination with Gefitinib (Iressa, ZD1839). Biochem. Pharmacol. 68, 135–144 (2004).
Xu, J. M. et al. Characterization of sequence-dependent synergy between ZD1839 ('Iressa') and oxaliplatin. Biochem. Pharmacol. 66, 551–563 (2003).
Benhar, M., Engelberg, D. & Levitzki, A. Cisplatin-induced activation of the EGF receptor. Oncogene 21, 8723–8731 (2002).
Van Schaeybroeck, S. et al. Epidermal growth factor receptor activity determines response of colorectal cancer cells to gefitinib alone and in combination with chemotherapy. Clin. Cancer Res. 11, 7480–7489 (2005).
Sumitomo, M. et al. ZD1839 modulates paclitaxel response in renal cancer by blocking paclitaxel-induced activation of the epidermal growth factor receptor-extracellular signal-regulated kinase pathway. Clin. Cancer Res. 10, 794–801 (2004).
Abdelmohsen, K. et al. Doxorubicin induces EGF receptor-dependent downregulation of gap junctional intercellular communication in rat liver epithelial cells. Biol. Chem. 386, 217–223 (2005).
Ullrich, A. & Schlessinger, J. Signal transduction by receptors with tyrosine kinase activity. Cell 61, 203–212 (1990).
Feng, F.Y. et al. EGFR degradation: a novel mechanism of gemcitabine-induced cell death in head and neck cancer cell lines. In 14th SPORE investigators' workshop 154 (Baltimore, 2006).
Friedmann, B., Caplin, M., Hartley, J. A. & Hochhauser, D. Modulation of DNA repair in vitro after treatment with chemotherapeutic agents by the epidermal growth factor receptor inhibitor gefitinib (ZD1839). Clin. Cancer Res. 10, 6476–6486 (2004).
Friedmann, B. J. et al. Interaction of the epidermal growth factor receptor and the DNA-dependent protein kinase pathway following gefitinib treatment. Mol. Cancer Ther. 5, 209–218 (2006).
Magne, N. et al. Molecular mechanisms underlying the interaction between ZD1839 ('Iressa') and cisplatin/5-fluorouracil. Br. J. Cancer 89, 585–592 (2003).
Giaccone, G. et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial — INTACT 1. J. Clin. Oncol. 22, 777–784 (2004).
Herbst, R. S. et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial — INTACT 2. J. Clin. Oncol. 22, 785–794 (2004).
Herbst, R. S. et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 23, 5892–5899 (2005).
Burtness, B., Goldwasser, M. A., Flood, W., Mattar, B. & Forastiere, A. A. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J. Clin. Oncol. 23, 8646–8654 (2005).
Moore, M. J. Brief communication: a new combination in the treatment of advanced pancreatic cancer. Semin. Oncol. 32, 5–6 (2005).
Moore, M. J. et al. Erlotinib plus gemcitabine compared to gemcitabine alone in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group [NCIC-CTG]. J. Clin. Oncol. 23, 1S–1S (2005).
Tsao, M. S. et al. Erlotinib in lung cancer- molecular and clinical predictors of outcome. N. Engl. J. Med. 353, 133–144 (2005).
Chung, K. Y. et al. Cetuximab shows activity in colourectal cancer patients with tumours that do not express the epidermal growth factor receptor by immunohistochemistry. J. Clin. Oncol. 23, 1803–1810 (2005).
Elie, C. et al. Lack of relationship between EGFR-1 immunohistochemical expression and prognosis in a multicentre clinical trial of 93 patients with advanced primary ovarian epithelial cancer (GINECO group). Br. J. Cancer 91, 470–475 (2004).
Segaert, S. et al. The management of skin reactions in cancer patients receiving epidermal growth factor receptor targeted therapies. J. Dtsch Dermatol. Ges. 3, 599–606 (2005).
Baselga, J. Combining the anti-EGFR agent gefitinib with chemotherapy in non-small-cell lung cancer: How do we go from INTACT to impact? J. Clin. Oncol. 22, 759–761 (2004).
Tan, A. R. et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J. Clin. Oncol. 22, 3080–3090 (2004).
Laux, I., Jain, A., Singh, S. & Agus, D. B. Epidermal growth factor receptor dimerization status determines skin toxicity to HER-kinase targeted therapies. Br. J. Cancer 94, 85–92 (2006).
Bowman, T., Garcia, R., Turkson, J. & Jove, R. STATs in oncogenesis. Oncogene 19, 2474–2488 (2000).
Wendel, H. G. et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 428, 332–337 (2004).
Hennessy, B. T., Smith, D. L., Ram, P. T., Lu, Y. & Mills, G. B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nature Rev. Drug Discov. 4, 988–1004 (2005).
Nishinaka, T. & Yabe-Nishimura, C. EGF receptor-ERK pathway is the major signalling pathway that mediates upregulation of aldose reductase expression under oxidative stress. Free Radic. Biol. Med. 31, 205–216 (2001).
Sebolt-Leopold, J. S. & Herrera, R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nature Rev. Cancer 4, 937–947 (2004).
Oliva, J. L., Griner, E. M. & Kazanietz, M. G. PKC isozymes and diacylglycerol-regulated proteins as effectors of growth factor receptors. Growth Factors 23, 245–252 (2005).
Huse, M. & Kuriyan, J. The conformational plasticity of protein kinases. Cell 109, 275–282 (2002).
Russo, M. W., Lukas, T. J., Cohen, S. & Staros, J. V. Identification of residues in the nucleotide binding site of the epidermal growth factor receptor/kinase. J. Biol. Chem. 260, 5205–5208 (1985).
Gullick, W. J., Downward, J., Foulkes, J. G. & Waterfield, M. D. Antibodies to the ATP-binding site of the human epidermal growth factor (EGF) receptor as specific inhibitors of EGF-stimulated protein-tyrosine kinase activity. Eur. J. Biochem. 158, 245–253 (1986).
Biscardi, J. S. et al. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 274, 8335–8343 (1999).
Grovdal, L. M., Stang, E., Sorkin, A. & Madshus, I. H. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp. Cell Res. 300, 388–395 (2004).
Buday, L. & Downward, J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell 73, 611–620 (1993).
Keilhack, H. et al. Phosphotyrosine 1173 mediates binding of the protein-tyrosine phosphatase SHP-1 to the epidermal growth factor receptor and attenuation of receptor signalling. J. Biol. Chem. 273, 24839–24846 (1998).
Kobayashi, S. et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 352, 786–792 (2005).
Choong, N. W. et al. Gefitinib response of erlotinib-refractory lung cancer involving meninges--role of EGFR mutation. Nature Clin. Pract. Oncol. 3, 50–57 (2006).
Bell, D. W. et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nature Genet. 37, 1315–1316 (2005).
Dittmann, K., Mayer, C. & Rodemann, H. P. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother. Oncol. 76, 157–161 (2005).
Boerner, J. L., Biscardi, J. S., Silva, C. M. & Parsons, S. J. Transactivating agonists of the EGF receptor require Tyr 845 phosphorylation for induction of DNA synthesis. Mol. Carcinog. 44, 262–273 (2005).
Boerner, J. L., Demory, M. L., Silva, C. & Parsons, S. J. Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol. Cell Biol. 24, 7059–7071 (2004).
Goldberg, R. M. Cetuximab. Nature Rev. Drug Discov. Suppl., S10–S11 (2005).
Kies, M. S. & Harari, P. M. Cetuximab (Imclone/Merck/Bristol-Myers Squibb). Curr. Opin. Investig. Drugs 3, 1092–1100 (2002).
Rowinsky, E. K. et al. Safety, pharmacokinetics, and activity of ABX-EGF, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer. J. Clin. Oncol. 22, 3003–3015 (2004).
Yang, X. D., Jia, X. C., Corvalan, J. R., Wang, P. & Davis, C. G. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit. Rev. Oncol. Hematol. 38, 17–23 (2001).
Ranson, M. Technology evaluation: ABX-EGF, Abgenix/Amgen. Curr. Opin. Mol. Ther. 5, 541–546 (2003).
Vanhoefer, U. et al. Phase I study of the humanized antiepidermal growth factor receptor monoclonal antibody EMD72000 in patients with advanced solid tumours that express the epidermal growth factor receptor. J. Clin. Oncol. 22, 175–184 (2004).
Kollmannsberger, C. et al. A phase I study of the humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody EMD 72000 (matuzumab) in combination with paclitaxel in patients with EGFR-positive advanced non-small-cell lung cancer (NSCLC). Ann. Oncol. (2006).
Modjtahedi, H. et al. Phase 1 trial and tumour localization of the anti-EGFR monoclonal antibody ICR62 in head and neck or lung cancer. Br. J. Cancer 73, 228–235 (1996).
Nakagawa, K. et al. Phase I pharmacokinetic trial of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor gefitinib ('Iressa', ZD1839) in Japanese patients with solid malignant tumours. Ann. Oncol. 14, 922–930 (2003).
Cohen, M. H., Johnson, J. R., Chen, Y. F., Sridhara, R. & Pazdur, R. FDA drug approval summary: erlotinib (Tarceva) tablets. Oncologist 10, 461–466 (2005).
Hoekstra, R. et al. Phase I and pharmacologic study of PKI166, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. Clin. Cancer Res. 11, 6908–6915 (2005).
Nemunaitis, J. et al. Phase 1 clinical and pharmacokinetics evaluation of oral CI-1033 in patients with refractory cancer. Clin. Cancer Res. 11, 3846–3853 (2005).
Calvo, E. et al. Administration of CI-1033, an irreversible pan-erbB tyrosine kinase inhibitor, is feasible on a 7-day on, 7-day off schedule: a phase I pharmacokinetic and food effect study. Clin. Cancer Res. 10, 7112–7120 (2004).
Campos, S. et al. Multicenter, randomized phase II trial of oral CI-1033 for previously treated advanced ovarian cancer. J. Clin. Oncol. 23, 5597–5604 (2005).
Erlichman, C. et al. EKB-569, an irreversible inhibitor of the epidermal growth factor recepton: Phase 1 trial results in patients with advanced solid tumours. Eur. J. Cancer 38, S64–S64 (2002).
Yoshimura, N. et al. EKB-569, a new irreversible epidermal growth factor receptor tyrosine kinase inhibitor, with clinical activity in patients with non-small cell lung cancer with acquired resistance to gefitinib. Lung Cancer 51, 363–368 (2006).
Burris, H. A., et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J. Clin. Oncol. 23, 5305–5313 (2005).
Acknowledgements
The work described in this paper was supported by US National Cancer Institute grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
University of Michigan Department of Radiation Oncology hompage
Rights and permissions
About this article
Cite this article
Nyati, M., Morgan, M., Feng, F. et al. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer 6, 876–885 (2006). https://doi.org/10.1038/nrc1953
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc1953
This article is cited by
-
Electron transfer-triggered imaging of EGFR signaling activity
Nature Communications (2022)
-
Recent advances and challenges of bispecific antibodies in solid tumors
Experimental Hematology & Oncology (2021)
-
Loss of DLX3 tumor suppressive function promotes progression of SCC through EGFR–ERBB2 pathway
Oncogene (2021)
-
Stapled EGFR peptide reduces inflammatory breast cancer and inhibits additional HER-driven models of cancer
Journal of Translational Medicine (2019)
-
FBXW2 suppresses migration and invasion of lung cancer cells via promoting β-catenin ubiquitylation and degradation
Nature Communications (2019)