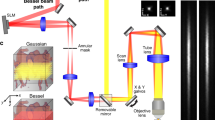
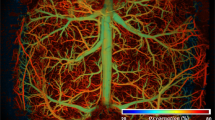
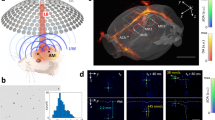
Abstract
Cerebrovascular dysfunction has an important role in the pathogenesis of multiple brain disorders. Measurement of hemodynamic responses in vivo can be challenging, particularly as techniques are often not described in sufficient detail and vary between laboratories. We present a set of standardized in vivo protocols that describe high-resolution two-photon microscopy and intrinsic optical signal (IOS) imaging to evaluate capillary and arteriolar responses to a stimulus, regional hemodynamic responses, and oxygen delivery to the brain. The protocol also describes how to measure intrinsic NADH fluorescence to understand how blood O2 supply meets the metabolic demands of activated brain tissue, and to perform resting-state absolute oxygen partial pressure (pO2) measurements of brain tissue. These methods can detect cerebrovascular changes at far higher resolution than MRI techniques, although the optical nature of these techniques limits their achievable imaging depths. Each individual procedure requires 1–2 h to complete, with two to three procedures typically performed per animal at a time. These protocols are broadly applicable in studies of cerebrovascular function in healthy and diseased brain in any of the existing mouse models of neurological and vascular disorders. All these procedures can be accomplished by a competent graduate student or experienced technician, except the two-photon measurement of absolute pO2 level, which is better suited to a more experienced, postdoctoral-level researcher.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Kety, S.S. The general metabolism of the brain in vivo. in Metabolism of the Nervous System 221–237 (ed. D. Richter) (Elsevier, 1957).
Sokoloff, L. The metabolism of the central nervous system in vivo. in Handbook of Physiology, Section I, Neurophysiology (eds. Field, J., Magoun, H. W. & Hall, V. E.) 3, 1843–1864 (American Physiological Society, 1960).
Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 (2011).
Sweeney, M.D., Sagare, A.P. & Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Iadecola, C. The pathobiology of vascular dementia. Neuron 80, 844–866 (2013).
Kisler, K., Nelson, A.R., Montagne, A. & Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 18, 419–434 (2017).
Arvanitakis, Z., Capuano, A.W., Leurgans, S.E., Bennett, D.A. & Schneider, J.A. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 15, 934–943 (2016).
Iturria-Medina, Y. et al. Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat. Commun. 7, 11934 (2016).
Montagne, A. et al. Brain imaging of neurovascular dysfunction in Alzheimer's disease. Acta Neuropathol. (Berl.) 131, 687–707 (2016).
Montagne, A., Zhao, Z. & Zlokovic, B.V. Alzheimer's disease: a matter of blood–brain barrier dysfunction? J. Exp. Med. 214, 3151–3169 (2017).
Murphy, M.J. et al. Widespread cerebral haemodynamics disturbances occur early in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 13, 202–209 (2012).
Ishikawa, T., Morita, M. & Nakano, I. Constant blood flow reduction in premotor frontal lobe regions in ALS with dementia - a SPECT study with 3D-SSP. Acta Neurol. Scand. 116, 340–344 (2007).
Yamashita, T. et al. Flow-metabolism uncoupling in the cervical spinal cord of ALS patients. Neurol. Sci. 38, 659–665 (2017).
Verfaillie, S.C.J. et al. Cerebral perfusion and glucose metabolism in Alzheimer's disease and frontotemporal dementia: two sides of the same coin? Eur. Radiol. 25, 3050–3059 (2015).
Malek, N. et al. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson's disease. Mov. Disord. 31, 1518–1526 (2016).
Al-Bachari, S., Vidyasagar, R., Emsley, H.C. & Parkes, L.M. Structural and physiological neurovascular changes in idiopathic Parkinson's disease and its clinical phenotypes. J. Cereb. Blood Flow Metab. 37, 3409–3421 (2017).
Drouin-Ouellet, J. et al. Cerebrovascular and blood-brain barrier impairments in Huntington's disease: potential implications for its pathophysiology. Ann. Neurol. 78, 160–177 (2015).
Chen, J.J., Salat, D.H. & Rosas, H.D. Complex relationships between cerebral blood flow and brain atrophy in early Huntington's disease. NeuroImage 59, 1043–1051 (2012).
Wardlaw, J.M., Smith, C. & Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 12, 483–497 (2013).
Wardlaw, J.M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838 (2013).
Montine, T.J. et al. Recommendations of the Alzheimer's disease-related dementias conference. Neurology 83, 851–860 (2014).
Snyder, H.M. et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement. 11, 710–717 (2015).
Hachinski, V. & World Stroke Organization Stroke and potentially preventable dementias proclamation: updated World Stroke Day Proclamation. Stroke 46, 3039–3040 (2015).
Faraco, G. & Iadecola, C. Hypertension: a harbinger of stroke and dementia. Hypertension 62, 810–817 (2013).
Last, D. et al. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care 30, 1193–1199 (2007).
Ingrisch, M. et al. Quantification of perfusion and permeability in multiple sclerosis: dynamic contrast-enhanced MRI in 3D at 3T. Invest. Radiol. 47, 252–258 (2012).
De Roos, A., van der Grond, J., Mitchell, G. & Westenberg, J. Magnetic resonance imaging of cardiovascular function and the brain: is dementia a cardiovascular-driven disease? Circulation 135, 2178–2195 (2017).
Qiu, C. & Fratiglioni, L. A major role for cardiovascular burden in age-related cognitive decline. Nat. Rev. Cardiol. 12, 267–277 (2015).
Lok, J. et al. Targeting the neurovascular unit in brain trauma. CNS Neurosci. Ther. 21, 304–308 (2015).
Toth, P. et al. Traumatic brain injury-induced autoregulatory dysfunction and spreading depression-related neurovascular uncoupling: pathomechanisms, perspectives, and therapeutic implications. Am. J. Physiol. Heart Circ. Physiol. 311, H1118–H1131 (2016).
Najjar, S. et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to Schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front. Psychiatry 8, 83 (2017).
Ances, B., Vaida, F., Ellis, R. & Buxton, R. Test-retest stability of calibrated BOLD-fMRI in HIV- and HIV+ subjects. NeuroImage 54, 2156–2162 (2011).
Iadecola, C. et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat. Neurosci. 2, 157–161 (1999).
Niwa, K. et al. Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc. Natl. Acad. Sci. USA 97, 9735–9740 (2000).
Chow, N. et al. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer's phenotype. Proc. Natl. Acad. Sci. USA 104, 823–828 (2007).
Miyazaki, K. et al. Early and progressive impairment of spinal blood flow—glucose metabolism coupling in motor neuron degeneration of ALS model mice. J. Cereb. Blood Flow Metab. 32, 456–467 (2012).
Kleinberger, G. et al. The FTD-like syndrome causing TREM2 T66M mutation impairs microglia function, brain perfusion, and glucose metabolism. EMBO J. 36, 1837–1853 (2017).
Toth, P. et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J. Cereb. Blood Flow Metab. 33, 1732–1742 (2013).
Faraco, G. et al. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J. Cereb. Blood Flow Metab. 36, 241–252 (2016).
Kisler, K. et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 20, 406–416 (2017).
Kim, T.N. et al. Line-scanning particle image velocimetry: an optical approach for quantifying a wide range of blood flow speeds in live animals. PLoS One 7, e38590 (2012).
Harrison, T.C., Sigler, A. & Murphy, T.H. Simple and cost-effective hardware and software for functional brain mapping using intrinsic optical signal imaging. J. Neurosci. Methods 182, 211–218 (2009).
Hillman, E.M.C. Optical brain imaging in vivo: techniques and applications from animal to man. J. Biomed. Opt. 12, 051402 (2007).
Sirotin, Y.B., Hillman, E.M.C., Bordier, C. & Das, A. Spatiotemporal precision and hemodynamic mechanism of optical point spreads in alert primates. Proc. Natl. Acad. Sci. USA 106, 18390–18395 (2009).
Frostig, R.D., Lieke, E.E., Ts'o, D.Y. & Grinvald, A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc. Natl. Acad. Sci. USA 87, 6082–6086 (1990).
Kasischke, K.A., Vishwasrao, H.D., Fisher, P.J., Zipfel, W.R. & Webb, W.W. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 305, 99–103 (2004).
Mayevsky, A. & Rogatsky, G.G. Mitochondrial function in vivo evaluated by NADH fluorescence: from animal models to human studies. Am. J. Physiol. Cell Physiol. 292, C615–C640 (2006).
Finikova, O.S. et al. Oxygen microscopy by two-photon-excited phosphorescence. Chemphyschem 9, 1673–1679 (2008).
Gama Sosa, M.A. et al. Age-related vascular pathology in transgenic mice expressing Presenilin 1-associated familial Alzheimer's disease mutations. Am. J. Pathol. 176, 353–368 (2010).
Blair, L.J. et al. Tau depletion prevents progressive blood-brain barrier damage in a mouse model of tauopathy. Acta Neuropathol. Commun. 3 (2015).
Lewis, J. et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 25, 402–405 (2000).
Bell, R.D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012).
Alata, W., Ye, Y., St-Amour, I., Vandal, M. & Calon, F. Human apolipoprotein Ež4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J. Cereb. Blood Flow Metab. 35, 86–94 (2015).
Nelson, A.R., Sweeney, M.D., Sagare, A.P. & Zlokovic, B.V. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer's disease. Biochim. Biophys. Acta 1862, 887–900 (2016).
Park, L. et al. Brain and circulating levels of A 1-40 differentially contribute to vasomotor dysfunction in the mouse brain. Stroke 44, 198–204 (2013).
Poliakova, T., Levin, O., Arablinskiy, A., Vasenina, E. & Zerr, I. Cerebral microbleeds in early Alzheimer's disease. J. Neurol. 263, 1961–1968 (2016).
Manaenko, A., Chen, H., Zhang, J.H. & Tang, J. Comparison of different preclinical models of intracerebral hemorrhage. in Intracerebral Hemorrhage Research (eds. Zhang, J. & Colohan, A.) 111, 9–14 (Springer, 2011).
Petraglia, A.L., Marky, A.H., Walker, C., Thiyagarajan, M. & Zlokovic, B.V. Activated protein C is neuroprotective and mediates new blood vessel formation and neurogenesis after controlled cortical impact. Neurosurgery 66, 165–171 (2010).
Toda, N. & Okamura, T. Hyperhomocysteinemia impairs regional blood flow: involvements of endothelial and neuronal nitric oxide. Pflugers Arch. 468, 1517–1525 (2016).
Sudduth, T.L., Powell, D.K., Smith, C.D., Greenstein, A. & Wilcock, D.M. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J. Cereb. Blood Flow Metab. 33, 708–715 (2013).
Hardigan, T., Hernandez, C., Ward, R., Hoda, M.N. & Ergul, A. TLR2 knockout protects against diabetes-mediated changes in cerebral perfusion and cognitive deficits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R927–R937 (2017).
Iordanova, B., Li, L., Clark, R.S.B. & Manole, M.D. Alterations in cerebral blood flow after resuscitation from cardiac arrest. Front. Pediatr. 5, 174 (2017).
Khan, M.B. et al. Chronic remote ischemic conditioning is cerebroprotective and induces vascular remodeling in a VCID model. Transl. Stroke Res. 9, 51–63 (2017).
Zhao, Z., Nelson, A.R., Betsholtz, C. & Zlokovic, B.V. Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078 (2015).
Winkler, E.A. et al. GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 18, 521–530 (2015).
Guemez-Gamboa, A. et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat. Genet. 47, 809–813 (2015).
Ben-Zvi, A. et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507–511 (2014).
Lacombe, P., Oligo, C., Domenga, V., Tournier-Lasserve, E. & Joutel, A. Impaired cerebral vasoreactivity in a transgenic mouse model of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy arteriopathy. Stroke 36, 1053–1058 (2005).
Joutel, A. et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J. Clin. Invest. 120, 433–445 (2010).
Sharma, A. & Shiras, A. Cancer stem cell-vascular endothelial cell interactions in glioblastoma. Biochem. Biophys. Res. Commun. 473, 688–692 (2016).
Dai, M., Yang, Y. & Shi, X. Lactate dilates cochlear capillaries via type V fibrocyte-vessel coupling signaled by nNOS. Am. J. Physiol. Heart Circ. Physiol. 301, H1248–H1254 (2011).
Martinez Sosa, S. & Smith, K.J. Understanding a role for hypoxia in lesion formation and location in the deep and periventricular white matter in small vessel disease and multiple sclerosis. Clin. Sci. Lond. 131, 2503–2524 (2017).
Tang, P. et al. In vivo two-photon imaging of axonal dieback, blood flow, and calcium influx with methylprednisolone therapy after spinal cord injury. Sci. Rep. 5, 9691 (2015).
Montagne, A. et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat. Med. 24, 326–337 (2018).
Uhlirova, H. et al. Cell type specificity of neurovascular coupling in cerebral cortex. eLife 5 e14315 (2016).
Kasischke, K.A. et al. Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions. J. Cereb. Blood Flow Metab. 31, 68–81 (2011).
Sakadži´, S. et al. Large arteriolar component of oxygen delivery implies a safe margin of oxygen supply to cerebral tissue. Nat. Commun. 5, 5734 (2014).
Shahram, M. & Milanfar, P. Imaging below the diffraction limit: a statistical analysis. IEEE Trans. Image Process. 13, 677–689 (2004).
Ram, S., Ward, E.S. & Ober, R.J. Beyond Rayleigh's criterion: a resolution measure with application to single-molecule microscopy. Proc. Natl. Acad. Sci. USA 103, 4457–4462 (2006).
Rayleigh, L. XXXI: Investigations in optics, with special reference to the spectroscope. Philos. Mag. Ser. 5 8, 261–274 (1879).
Nyquist, H. Certain topics in telegraph transmission theory. Trans. Am. Inst. Electr. Eng. 47, 617–644 (1928).
Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423 (1948).
Pawley, J.B. Points, pixels, and gray levels: digitizing image data. in Handbook of Biological Confocal Microscopy (ed. Pawley, J.B.) 59–79 (Springer, 2006).
Hall, C.N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014).
Mishra, A. et al. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 19, 1619–1627 (2016).
Otsu, Y. et al. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat. Neurosci. 18, 210–218 (2015).
Kornfield, T.E. & Newman, E.A. Regulation of blood flow in the retinal trilaminar vascular network. J. Neurosci. 34, 11504–11513 (2014).
Biesecker, K.R. et al. Glial cell calcium signaling mediates capillary regulation of blood flow in the retina. J. Neurosci. 36, 9435–9445 (2016).
Drew, P.J., Shih, A.Y. & Kleinfeld, D. Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity. Proc. Natl. Acad. Sci. USA 108, 8473–8478 (2011).
Takano, T. et al. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 9, 260–267 (2006).
Hill, R.A. et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 87, 95–110 (2015).
Damisah, E.C., Hill, R.A., Tong, L., Murray, K.N. & Grutzendler, J. A fluoro-Nissl dye identifies pericytes as distinct vascular mural cells during in vivo brain imaging. Nat. Neurosci. 20, 1023–1032 (2017).
Wei, H.S. et al. Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron 91, 851–862 (2016).
Chang, C.-I., Du, Y., Wang, J., Guo, S.-M. & Thouin, P.D. Survey and comparative analysis of entropy and relative entropy thresholding techniques. IEE Proc. - Vis. Image Signal Process. 153, 837 (2006).
Drew, P.J., Blinder, P., Cauwenberghs, G., Shih, A.Y. & Kleinfeld, D. Rapid determination of particle velocity from space-time images using the Radon transform. J. Comput. Neurosci. 29, 5–11 (2010).
Summers, P.M., Taylor, Z.J. & Shih, A.Y. Two-photon imaging of cerebral vasodynamics in awake mice during health and disease. in Advances in Intravital Microscopy (ed. Weigert, R.) 25–43 (Springer, 2014).
Art, J. Photon detectors for confocal microscopy. in Handbook of Biological Confocal Microscopy (ed. Pawley, J.B.) 251–264 (Springer, 2006).
Baran, U. & Wang, R.K. Review of optical coherence tomography based angiography in neuroscience. Neurophotonics 3, 010902 (2016).
Srinivasan, V.J. et al. OCT methods for capillary velocimetry. Biomed. Opt. Express 3, 612–629 (2012).
Ma, Y. et al. Wide-field optical mapping of neural activity and brain haemodynamics: considerations and novel approaches. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150360 (2016).
Wang, Y. et al. 3K3A-activated protein C stimulates postischemic neuronal repair by human neural stem cells in mice. Nat. Med. 22, 1050–1055 (2016).
Berndt, N., Kann, O. & Holzhütter, H.-G. Physiology-based kinetic modeling of neuronal energy metabolism unravels the molecular basis of NAD(P)H fluorescence transients. J. Cereb. Blood Flow Metab. 35, 1494–1506 (2015).
Yaseen, M.A. et al. In vivo imaging of cerebral energy metabolism with two-photon fluorescence lifetime microscopy of NADH. Biomed. Opt. Express 4, 307–321 (2013).
Yaseen, M.A. et al. Fluorescence lifetime microscopy of NADH distinguishes alterations in cerebral metabolism in vivo. Biomed. Opt. Express 8, 2368–2385 (2017).
Baraghis, E. et al. Two-photon microscopy of cortical NADH fluorescence intensity changes: correcting contamination from the hemodynamic response. J. Biomed. Opt. 16, 106003 (2011).
Zhao, Y. et al. In vivo monitoring of cellular energy metabolism using SoNar, a highly responsive sensor for NAD+/NADH redox state. Nat. Protoc. 11, 1345–1359 (2016).
Mongeon, R., Venkatachalam, V. & Yellen, G. Cytosolic NADH-NAD(+) redox visualized in brain slices by two-photon fluorescence lifetime biosensor imaging. Antioxid. Redox Signal. 25, 553–563 (2016).
Vanderkooi, J.M., Maniara, G., Green, T.J. & Wilson, D.F. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J. Biol. Chem. 262, 5476–5482 (1987).
Becker, W. Fluorescence lifetime imaging - techniques and applications: fluorescence lifetime imaging. J. Microsc. 247, 119–136 (2012).
Sakadzi´, S. et al. Simultaneous imaging of cerebral partial pressure of oxygen and blood flow during functional activation and cortical spreading depression. Appl. Opt. 48, D169–D177 (2009).
Wilson, D.F. et al. Effect of hyperventilation on oxygenation of the brain cortex of newborn piglets. J. Appl. Physiol. 70, 2691–2696 (1991).
Wilson, D.F., Gomi, S., Pastuszko, A. & Greenberg, J.H. Microvascular damage in the cortex of cat brain from middle cerebral artery occlusion and reperfusion. J. Appl. Physiol. 74, 580–589 (1993).
Yaseen, M.A. et al. Optical monitoring of oxygen tension in cortical microvessels with confocal microscopy. Opt. Express 17, 22341–22350 (2009).
Sakadzi´, S. et al. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat. Methods 7, 755–759 (2010).
Lecoq, J. et al. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nat. Med. 17, 893–898 (2011).
Devor, A. et al. 'Overshoot' of O2 is required to maintain baseline tissue oxygenation at locations distal to blood vessels. J. Neurosci. 31, 13676–13681 (2011).
Lyons, D.G., Parpaleix, A., Roche, M. & Charpak, S. Mapping oxygen concentration in the awake mouse brain. eLife 5, e12024 (2016).
Kazmi, S.M.S. et al. Three-dimensional mapping of oxygen tension in cortical arterioles before and after occlusion. Biomed. Opt. Express 4, 1061 (2013).
Spencer, J.A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014).
Yaseen, M.A. et al. Multimodal optical imaging system for in vivo investigation of cerebral oxygen delivery and energy metabolism. Biomed. Opt. Express 6, 4994–5007 (2015).
Kalmbach, A.S. & Waters, J. Brain surface temperature under a craniotomy. J. Neurophysiol. 108, 3138–3146 (2012).
Shirey, M.J. et al. Brief anesthesia, but not voluntary locomotion, significantly alters cortical temperature. J. Neurophysiol. 114, 309–322 (2015).
Goldey, G.J. et al. Removable cranial windows for long-term imaging in awake mice. Nat. Protoc. 9, 2515–2538 (2014).
Rumsey, W.L., Vanderkooi, J.M. & Wilson, D.F. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science 241, 1649–1651 (1988).
Mik, E.G., van Leeuwen, T.G., Raat, N.J. & Ince, C. Quantitative determination of localized tissue oxygen concentration in vivo by two-photon excitation phosphorescence lifetime measurements. J. Appl. Physiol. 97, 1962–1969 (2004).
Holtmaat, A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1144 (2009).
Polesskaya, O. et al. Detection of microregional hypoxia in mouse cerebral cortex by two-photon imaging of endogenous NADH fluorescence. J. Vis. Exp. (60) (2012).
Shih, A.Y., Mateo, C., Drew, P.J., Tsai, P.S. & Kleinfeld, D. A polished and reinforced thinned-skull window for long-term imaging of the mouse brain. J. Vis. Exp. 61, 3742 (2012).
Tallquist, M.D., French, W.J. & Soriano, P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 1, E52 (2003).
Schneider, C.A., Rasband, W.S. & Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Sakadži´, S. et al. Cerebral blood oxygenation measurement based on oxygen-dependent quenching of phosphorescence. J. Vis. Exp. (2011), (51).
Sinks, L.E. et al. Two-photon microscopy of oxygen: polymersomes as probe carrier vehicles. J. Phys. Chem. B 114, 14373–14382 (2010).
Acknowledgements
This work was supported by US National Institutes of Health grants R01AG023084, R01NS090904, R01NS034467, R01AG039452, R01NS100459, and P01AG052350 to B.V.Z.; grants R24NS092986, R01EB018464, and R01NS091230 to S.S., S.A.V., and D.A.B.; by funding from the Alzheimer's Association and Cure Alzheimer's fund to B.V.Z.; and by funding from the Fondation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease (ref. no. 16 CVD 05) to B.V.Z. We thank R. Jaswal for helping to create Figure 8. We gratefully acknowledge the feedback, forum posts, and questions from our peers regarding the techniques presented here, which provided the inspiration for the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
K.K. and B.V.Z. conceived the concept presented here. K.K., S.S., and B.V.Z. contributed to the experimental design. K.K. and S.S. performed experiments and analyzed the data. D.L. and M.D.S. performed experiments and contributed to cranial window protocol development, and S.P. and M.E.K. contributed to PtP-C343 dye development. D.A.B. and S.A.V. contributed to the project design. B.V.Z. contributed to the project design and supervised the project. K.K., S.S., and B.V.Z. wrote the manuscript with input from all authors. S.S. and B.V.Z. shared senior authorship responsibilities.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1. (PDF 180 kb)
Rights and permissions
About this article
Cite this article
Kisler, K., Lazic, D., Sweeney, M. et al. In vivo imaging and analysis of cerebrovascular hemodynamic responses and tissue oxygenation in the mouse brain. Nat Protoc 13, 1377–1402 (2018). https://doi.org/10.1038/nprot.2018.034
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2018.034
This article is cited by
-
Blockage of VEGF function by bevacizumab alleviates early-stage cerebrovascular dysfunction and improves cognitive function in a mouse model of Alzheimer’s disease
Translational Neurodegeneration (2024)
-
Deep optoacoustic localization microangiography of ischemic stroke in mice
Nature Communications (2023)
-
A Through-Intact-Skull (TIS) chronic window technique for cortical structure and function observation in mice
eLight (2022)
-
Multi-scale optoacoustic molecular imaging of brain diseases
European Journal of Nuclear Medicine and Molecular Imaging (2021)
-
Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss
Nature Neuroscience (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.