Abstract
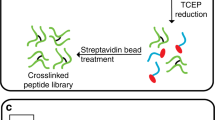
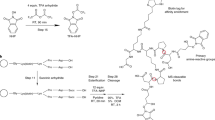
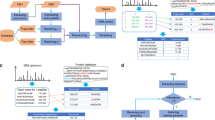
This protocol describes a workflow for creating structural models of proteins or protein complexes using distance restraints derived from cross-linking mass spectrometry experiments. The distance restraints are used (i) to adjust preliminary models that are calculated on the basis of a homologous template and primary sequence, and (ii) to select the model that is in best agreement with the experimental data. In the case of protein complexes, the cross-linking data are further used to dock the subunits to one another to generate models of the interacting proteins. Predicting models in such a manner has the potential to indicate multiple conformations and dynamic changes that occur in solution. This modeling protocol is compatible with many cross-linking workflows and uses open-source programs or programs that are free for academic users and do not require expertise in computational modeling. This protocol is an excellent additional application with which to use cross-linking results for building structural models of proteins. The established protocol is expected to take 6–12 d to complete, depending on the size of the proteins and the complexity of the cross-linking data.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
25 June 2018
In the version of this article initially published online, the authors used incorrectly defined restraints for specifying the distance between residues when using the HADDOCK portal. Following the publication of a Correspondence by the developers of the HADDOCK portal (Nat. Protoc. https://dx.doi.org/10.1038/s41596-018-0017-6, 2018) and a Reply by the authors of the Protocol (Nat. Protoc. https://dx.doi.org/10.1038/s41596-018-0018-5, 2018), the syntax in Step 21 has been corrected. In addition, the input files (available as Supplementary Data 5–7) have been replaced. This error has been corrected in the HTML and PDF versions of the article.
References
Berman, H.M. et al. The Protein Data Bank. Nucleic Acids Res. 28, 235–242 (2000).
Leitner, A., Faini, M., Stengel, F. & Aebersold, R. Crosslinking and mass spectrometry: an integrated technology to understand the structure and function of molecular machines. Trends Biochem. Sci. 41, 20–32 (2016).
Holding, A.N. XL-MS: protein cross-linking coupled with mass spectrometry. Methods 89, 54–63 (2015).
Herzog, F. et al. Structural probing of a protein phosphatase 2A network by chemical cross-linking and mass spectrometry. Science 337, 1348–1352 (2012).
Kahraman, A. et al. Cross-link guided molecular modeling with ROSETTA. PLoS One 8, e73411 (2013).
Robinson, P.J. et al. Molecular architecture of the yeast mediator complex. Elife 4, e08719 (2015).
Shi, Y. et al. Structural characterization by cross-linking reveals the detailed architecture of a coatomer-related heptameric module from the nuclear pore complex. Mol. Cell. Proteomics 13, 2927–2943 (2014).
Rampler, E. et al. Comprehensive cross-linking mass spectrometry reveals parallel orientation and flexible conformations of plant HOP2–MND1. J. Proteome Res. 14, 5048–5062 (2015).
Yang, J. et al. The I-TASSER suite: protein structure and function prediction. Nat. Methods 12, 7–8 (2015).
Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 9, 40 (2008).
de Vries, S.J., Van Dijk, M. & Bonvin, A.M. The HADDOCK web server for data-driven biomolecular docking. Nat. Protoc. 5, 883 (2010).
Dominguez, C., Boelens, R. & Bonvin, A.M. HADDOCK: a proteinprotein docking approach based on biochemical or biophysical information. J. Am. Chem. Soc. 125, 1731–1737 (2003).
Rodrigues, J.P. & Bonvin, A.M. Integrative computational modeling of protein interactions. FEBS J. 281, 1988–2003 (2014).
Zhang, Y. I-TASSER: fully automated protein structure prediction in CASP8. Proteins 77, 100–113 (2009).
De Vries, S.J. et al. HADDOCK versus HADDOCK: new features and performance of HADDOCK2. 0 on the CAPRI targets. Proteins 69, 726–733 (2007).
Leitner, A., Walzthoeni, T. & Aebersold, R. Lysine-specific chemical cross-linking of protein complexes and identification of cross-linking sites using LC-MS/MS and the xQuest/xProphet software pipeline. Nat. Protoc. 9, 120 (2014).
Schmidt, C. & Robinson, C.V. A comparative cross-linking strategy to probe conformational changes in protein complexes. Nat. Protoc. 9, 2224–2236 (2014).
Zorn, M., Ihling, C.H., Golbik, R., Sawers, R.G. & Sinz, A. Mapping cell envelope and periplasm protein interactions of Escherichia coli respiratory formate dehydrogenases by chemical cross-linking and mass spectrometry. J. Proteome Res. 13, 5524–5535 (2014).
Zheng, C. et al. Cross-linking measurements of in vivo protein complex topologies. Mol. Cell. Proteomics 10, M110.006841 (2011).
Lasker, K. et al. Integrative structure modeling of macromolecular assemblies from proteomics data. Mol. Cell. Proteomics 9, 1689–1702 (2010).
Russel, D. et al. Putting the pieces together: integrative modeling platform software for structure determination of macromolecular assemblies. PLoS Biol. 10, e1001244 (2012).
Müller, M.Q. et al. An innovative method to study target proteindrug interactions by mass spectrometry. J. Med. Chem. 52, 2875–2879 (2009).
Leitner, A. et al. Expanding the chemical cross-linking toolbox by the use of multiple proteases and enrichment by size exclusion chromatography. Mol. Cell. Proteomics 11, M111.014126 (2012).
Fritzsche, R., Ihling, C.H., Götze, M. & Sinz, A. Optimizing the enrichment of cross-linked products for mass spectrometric protein analysis. Rapid Commun. Mass Spectrom. 26, 653–658 (2012).
Kao, A. et al. Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes. Mol. Cell. Proteomics 10, M110. 002212 (2010).
Liu, F., Rijkers, D.T., Post, H. & Heck, A.J. Proteome-wide profiling of protein assemblies by cross-linking mass spectrometry. Nat. Methods 12, 1179–1184 (2015).
Müller, M.Q., Dreiocker, F., Ihling, C.H., Scha¨ fer, M. & Sinz, A. Cleavable cross-linker for protein structure analysis: reliable identification of cross-linking products by tandem MS. Anal. Chem. 82, 6958–6968 (2010).
Chakrabarty, J.K., Naik, A.G., Fessler, M.B., Munske, G.R. & Chowdhury, S.M. Differential tandem mass spectrometry-based cross-linker: a new approach for high confidence in identifying protein cross-linking. Anal. Chem. 88, 10215–10222 (2016).
Yu, C. et al. Developing a multiplexed quantitative cross-linking mass spectrometry platform for comparative structural analysis of protein complexes. Anal. Chem. 88, 10301–10308 (2016).
Yang, B. et al. Identification of cross-linked peptides from complex samples. Nat. Methods 9, 904–906 (2012).
Yilmaz, S. et al. Xilmass: a new approach toward the identification of cross-linked peptides. Anal. Chem. 88, 9949–9957 (2016).
Götze, M. et al. StavroX—a software for analyzing crosslinked products in protein interaction studies. J. Am. Soc. Mass Spectrom. 23, 76–87 (2012).
Lima, D.B. et al. SIM-XL: a powerful and user-friendly tool for peptide cross-linking analysis. J. Proteomics 129, 51–55 (2015).
Grimm, M., Zimniak, T., Kahraman, A. & Herzog, F. xVis: a web server for the schematic visualization and interpretation of crosslink-derived spatial restraints. Nucleic Acids Res. 43, W362–W369 (2015).
Combe, C.W., Fischer, L. & Rappsilber, J. xiNET: cross-link network maps with residue resolution. Mol. Cell. Proteomics 14, 1137–1147 (2015).
Riffle, M., Jaschob, D., Zelter, A. & Davis, T.N. ProXL (Protein Cross-Linking Database): a platform for analysis, visualization, and sharing of protein cross-linking mass spectrometry data. J. Proteome Res. 15, 2863–2870 (2016).
Erzberger, J.P. et al. Molecular architecture of the 40SeIF1eIF3 translation initiation complex. Cell 158, 1123–1135 (2014).
Politis, A. et al. A mass spectrometry-based hybrid method for structural modeling of protein complexes. Nat. Methods 11, 403–406 (2014).
Gaik, M. et al. Structural basis for assembly and function of the Nup82 complex in the nuclear pore scaffold. J. Cell Biol. 208, 283–297 (2015).
Ding, Y.-H. et al. Increasing the depth of mass-spectrometry-based structural analysis of protein complexes through the use of multiple cross-linkers. Anal. Chem. 88, 4461–4469 (2016).
Trahan, C. & Oeffinger, M. Targeted cross-linking-mass spectrometry determines vicinal interactomes within heterogeneous RNP complexes. Nucleic Acids Res. 44, 1354–1369 (2016).
Webb, B. & Sali, A. Protein structure modeling with MODELLER. in Protein Structure Prediction 1137, 1–15 (Humana Press, 2014).
Kuntal, B.K., Aparoy, P. & Reddanna, P. EasyModeller: a graphical interface to MODELLER. BMC Res. Notes 3, 226–226 (2010).
Matthew Allen Bullock, J., Schwab, J., Thalassinos, K. & Topf, M. The importance of non-accessible crosslinks and solvent accessible surface distance in modeling proteins with restraints from crosslinking mass spectrometry. Mol. Cell. Proteomics 15, 2491–2500 (2016).
Sinz, A. Divide and conquer: cleavable cross-linkers to study protein conformation and protein-protein interactions. Anal. Bioanal. Chem. 409, 33–44 (2017).
Tan, D. et al. Trifunctional cross-linker for mapping protein-protein interaction networks and comparing protein conformational states. Elife 5, 12509 (2016).
Kang, H.-A. et al. Crystal structure of Hop2–Mnd1 and mechanistic insights into its role in meiotic recombination. Nucleic Acids Res. 43, 3841–3856 (2015).
Kahraman, A., Malmström, L. & Aebersold, R. Xwalk: computing and visualizing distances in cross-linking experiments. Bioinformatics 27, 2163–2164 (2011).
de Vries, S.J. & Bonvin, A.M. CPORT: a consensus interface predictor and its performance in prediction-driven docking with HADDOCK. PLoS One 6, e17695 (2011).
Pettersen, E.F. et al. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Song, J.-G. et al. Structural insights into Ca2+-calmodulin regulation of Plectin 1a-Integrin β4 interaction in hemidesmosomes. Structure 23, 558–570 (2015).
Hall, Z., Schmidt, C. & Politis, A. Uncovering the early assembly mechanism for amyloidogenic beta2-Microglobulin using cross-linking and native mass spectrometry. J. Biol. Chem. 291, 4626–4637 (2016).
Acknowledgements
This research was funded by the Austrian Science Fund (SFB F3402, P24685-B24 and TRP 308-N15) and the EU FP7 project MEIOsys (222883-2). We thank the Research Institute of Molecular Pathology (IMP) and the Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA) for general funding. We acknowledge M. Hartl and T. Gossenreiter for useful and inspiring scientific discussions. Molecular graphics and analyses were performed with the UCSF Chimera package. Chimera was developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS P41-GM103311). Images in Chimera were created using Persistence of Vision Raytracer (v3.6), Persistence of Vision Pty. Ltd. (2004), and were retrieved from http://www.povray.org/download/.
Author information
Authors and Affiliations
Contributions
Z.O.-N., E.R., D.M.H., T.S. and K.M. designed and developed the modeling workflow. J.D. and O.H. performed data analysis and tested the workflow. Z.O.-N., R.B. and D.M.H. wrote and edited the manuscript. P.S. provided substantial input into the method development.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Data 1
I-TASSER prediction of bovine cytochrome c. Example input and output files, along with instructions for comparative modeling of bovine cytochrome C using cross-linking data (utilizes XL data originally published by Kao et al.25). (ZIP 250 kb)
Supplementary Data 2
I-TASSER prediction of HOP2. Example input and output files, along with instructions for comparative modeling of HOP2 from A. thaliana using cross-linking data (utilizes XL data originally published by Rampler et al.8). (ZIP 767 kb)
Supplementary Data 3
I-TASSER prediction of full length calmodulin. Example input and output files, along with instructions for comparative modeling of human calmodulin using cross-linking data (utilizes XL data originally published by Yilmaz et al.31). (ZIP 506 kb)
Supplementary Data 4
I-TASSER prediction of MND1. Example input and output files, along with instructions for comparative modeling of MND1 from A. thaliana using cross-linking data (utilizes XL data originally published by Rampler et al.8). (ZIP 639 kb)
Supplementary Data 5
HADDOCK docking of calmodulin to plectin. Example input and output files, along with instructions for protein–protein docking of human N-lobe of calmodulin and the actin-binding domain of mouse plectin using cross-linking data (utilizes XL data originally published by Yilmaz et al.31 and Song et al.51). (ZIP 6127 kb)
Supplementary Data 6
HADDOCK docking of PPP2R1A to PPP2CA. Example input and output files, along with instructions for protein–protein docking of mouse Ppp2r1a and human PPP2CA using cross-linking data (utilizes XL data originally published by Herzog et al. 4). (ZIP 6841 kb)
Supplementary Data 7
HADDOCK docking of HOP2 to MND1. Example input and output files, along with instructions for protein–protein docking of HOP2 and MND1 from A. thaliana using cross-linking data (utilizes XL data originally published by Rampler et al. 8). (ZIP 7835 kb)
Supplementary Data 8
Windows batch script that can be used to automatically run Xwalk on a series of PDB files. (TXT 1 kb)
Supplementary Data 9
Description of structure and content of supplementary data sets. It is recommended to read this file before using the supplementary data sets. (PDF 192 kb)
Rights and permissions
About this article
Cite this article
Orbán-Németh, Z., Beveridge, R., Hollenstein, D. et al. Structural prediction of protein models using distance restraints derived from cross-linking mass spectrometry data. Nat Protoc 13, 478–494 (2018). https://doi.org/10.1038/nprot.2017.146
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.146
This article is cited by
-
Structural insights into DNA N6-adenine methylation by the MTA1 complex
Cell Discovery (2023)
-
Integrative structural modeling of macromolecular complexes using Assembline
Nature Protocols (2022)
-
Systems structural biology measurements by in vivo cross-linking with mass spectrometry
Nature Protocols (2019)
-
Defining distance restraints in HADDOCK
Nature Protocols (2018)
-
Reply to ‘Defining distance restraints in HADDOCK’
Nature Protocols (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.