Abstract
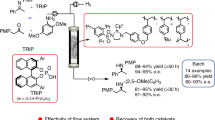
Organozinc reagents are versatile building blocks for introducing C(sp2)-C(sp3) and C(sp3)-C(sp3) bonds into organic structures. However, despite their ample synthetic versatility and broad functional group tolerance, the use of organozinc reagents in the laboratory is limited because of their instability, exothermicity and water sensitivity, as well as their labor-intensive preparation. Herein, we describe an on-demand synthesis of these useful reagents under continuous flow conditions, overcoming these primary limitations and supporting widespread adoption of these reagents in synthetic organic chemistry. To exemplify this procedure, a solution of ethyl zincbromoacetate is prepared by flowing ethyl bromoacetate through a column containing metallic zinc. The temperature of the column is controlled by a heating jacket and a thermocouple in close contact with it. Advice on how to perform the procedure using alternative equipment is also given to allow a wider access to the methodology. Here we describe the preparation of 50 ml of solution, which takes 1 h 40 min, although up to 250–300 ml can be prepared with the same column setup at a rate of 30 ml per h. The procedure provides the reagent as a clean solution with reproducible concentration. Organozinc solutions generated in flow can be coupled to a second flow reactor to perform a Reformatsky reaction or can be collected over a flask containing the required reagents for a batch Negishi reaction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout















Similar content being viewed by others
References
Roughley, S.D. & Jordan, A.M. The medicinal chemist's toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Brown, D.G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Tsukamoto, T. Tough times for medicinal chemists: are we to blame? ACS Med. Chem. Lett. 4, 369–370 (2013).
Walters, W.P., Green, J., Weiss, J.R. & Murcko, M.A.J. What do medicinal chemists actually make? A 50-year retrospective. J. Med. Chem. 54, 6405–4516 (2011).
Hirose, T. & Kodama, K. Recent advances in organozinc reagents. in Comprehensive Organic Synthesis 2nd edn, Vol. 1 (eds. Knochel, P. & Molander, G.A.) 204–266 (Elsevier, 2014).
Knochel, P. & Singer, R.D. Preparation and reactions of polyfunctional organozinc reagents in organic synthesis. Chem. Rev. 6, 2117–2188 (1993).
Rieke, R.D. & Kim, S.H. Organozinc reagents prepared from highly active zinc. e-EROS Encyclopedia of Reagents for Organic Synthesis (Wiley, 2012).
Zhu, L., Wehmeyer, R.M. & Rieke, R.D. The direct formation of functionalized alkyl(aryl)zinc halides by oxidative addition of highly reactive zinc with organic halides and their reactions with acid chlorides, α,β-unsaturated ketones, and allylic, aryl, and vinyl halides. J. Org. Chem. 56, 1445–1453 (1991).
Zhu, L. & Rieke, R.D. A facile method for the preparation of functionalized 2,3-disubstituted-1,3-butadienes. Tetrahedron Lett. 32, 2865–2866 (1991).
Zhu, L., Shaughnessy, K.H. & Rieke, R.D. A facile method for the preparation of functionalized 2-halo-1-olefins. Synth. Commun. 23, 525–529 (1993).
Huck, L., Berton, M., de la Hoz, A., Díaz-Ortiz, A. & Alcázar, J. Reformatsky and Blaise reactions in flow as a tool for drug discovery. One pot diversity oriented synthesis of valuable intermediates and heterocycles. Green Chem. 19, 1420–1424 (2017).
Dilman, A.D. & Levin, V.V. Advances in the chemistry of organozinc reagents. Tetrahedron Lett. 57, 3986–3992 (2016).
Rieke, R.D., Hudnall, P.M. & Uhm, S.J. Activated metals. Preparation of highly reactive zinc. J. Chem. Soc. Chem. Commun. 269–270 (1973).
Soorukram, D., Boudet, N., Malakov, V. & Knochel, P. Preparation of polyfunctionalized 2,6-dimethoxypyrimidine derivatives via chemo- and regioselective direct zinc insertion. Synthesis 3915–3922 (2007).
Malet-Sanz, L. & Flavien, S. Continuous flow synthesis. A pharma perspective. J. Med. Chem. 55, 4062–4098 (2012).
Wegner, J., Ceylan, S. & Kirschning, A. Ten key issues in modern flow chemistry. Chem. Commun. 47, 4583–4592 (2011).
Noel, T., Kuhn, S., Musacchio, A.J., Jensen, K.F. & Buchwald, S.L. Suzuki-Miyaura cross-coupling reactions in flow: multistep synthesis enabled by a microfluidic extraction. Angew. Chem. Int. Ed. Engl. 50, 5943–5946 (2011).
Newman, S.G. & Jensen, K.F. The role of flow in green chemistry and engineering. Green Chem. 15, 1456–1472 (2013).
Alonso, N., Miller, L.Z., Muñoz, J.d.M., Alcázar, J. & McQuade, D.T. Continuous synthesis of organozinc halides coupled to Negishi reactions. Adv. Synth. Catal. 356, 3737–3741 (2014).
Yang, H. et al. Reaction of organozinc halides with aryl isocyanates. Tetrahedron 69, 2588–2593 (2013).
Knochel, P., Yeh, M.C.P., Berk, S.C. & Talbert, J. Synthesis and reactivity toward acyl chlorides and enones of the new highly functionalized copper reagents RCu(CN) ZnI. J. Org. Chem. 53, 2390–2392 (1988).
Mineno, M., Sawai, Y., Kanno, K., Sawada, N. & Mizufune, H. a rapid and diverse construction of 6-substituted-5,6-dihydro-4-hydroxy-2-pyrones through double Reformatsky reaction. Tetrahedron 69, 10921–10926 (2013).
Mineno, M., Sawai, Y., Kanno, K., Sawada, N. & Mizufune, H. Double Reformatsky reaction: divergent synthesis of δ-hydroxy-β-ketoesters. J. Org. Chem. 78, 5843–5850 (2013).
Greszler, S.N., Malinowski, J.T. & Johnson, J.S. Formal synthesis of leustroducsin B via Refortmasky/Claisen condensation of silyl glyoxylates. Org. Lett. 13, 3206–3209 (2011).
Greszler, S.N., Malinowski, J.T. & Johnson, J.S. Remote stereoinduction in the acylation of fully substituted enolates: tandem Reformatsky/quaternary Claysen condensations of silyl glyoxylates and β-lactones. J. Am. Chem. Soc. 132, 17393–17395 (2010).
Greszler, S.N. & Johnson, J.S. Diasteroselective synthesis of pentasubstituted γ-butyrolactones from silyl glyoxylates and ketones through a double Reformatsky reaction. Angew. Chem. Int. Ed. Engl. 48, 3689–3691 (2009).
Wong, B. et al. A chemoselective Reformatsky-Negishi approach to α-haloaryl esters. Tetrahedron 70, 1508–1515 (2014).
McCann, L.C. & Organ, M.G. On The remarkably different role of salt in the cross-coupling of arylzincs from that seen with alkylzincs. Angew. Chem. Int. Ed. Engl. 53, 4386–4389 (2014).
Feng, C., Cunningham, W.C., Easter, Q.T. & Blum, S.A. Role of LiCl in generating soluble organozinc reagent. J. Am. Chem. Soc. 138, 11156–11159 (2016).
Acknowledgements
We thank D.T. McQuade, Z. Miller, J. de Mata Muñoz, N. Alonso, A. de la Hoz and Á. Díaz Ortiz for their input in the progress of developing this methodology. We also thank O. Kappe for his advice.
Author information
Authors and Affiliations
Contributions
M.B. and L.H. carried out the experiments. All authors designed the protocol and J.A. supervised the project. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Methods
Preparation of ethyl 3-hydroxy-3-(3-ethoxyphenyl)butanoate and ethyl 2-(4-bromophenyl)acetate. (PDF 354 kb)
Rights and permissions
About this article
Cite this article
Berton, M., Huck, L. & Alcázar, J. On-demand synthesis of organozinc halides under continuous flow conditions. Nat Protoc 13, 324–334 (2018). https://doi.org/10.1038/nprot.2017.141
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.141
This article is cited by
-
Continuous Flow Generation of Highly Reactive Organometallic Intermediates: A Recent Update
Journal of Flow Chemistry (2024)
-
The applications of organozinc reagents in continuous flow chemistry: Negishi coupling
Journal of Flow Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.