Abstract
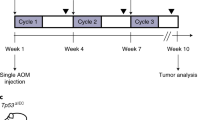
Most currently available colorectal cancer (CRC) mouse models are not suitable for studying progression toward the metastatic stage. Recently, establishment of tumor organoid lines, either from murine CRC models or patients, and the possibility of engineering them with genome-editing technologies, have provided a large collection of tumor material faithfully recapitulating phenotypic and genetic heterogeneity of native tumors. To study tumor progression in the natural in vivo environment, we developed an orthotopic approach based on transplantation of CRC organoids into the cecal epithelium. The 20-min procedure is described in detail here and enables growth of transplanted organoids into a single tumor mass within the intestinal tract. Due to long latency, tumor cells are capable of spreading through the blood circulation and forming metastases at distant sites. This method is designed to generate tumors suitable for studying CRC progression, thereby providing the opportunity to visualize tumor cell dynamics in vivo in real time by intravital microscopy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zauber, A.G. et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 366, 687–696 (2012).
Torre, L.A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Corbett, T.H., Griswold, D.P. Jr., Roberts, B.J., Peckham, J.C. & Schabel, F.M. Jr. Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 35, 2434–2439 (1975).
Moser, A.R., Pitot, H.C. & Dove, W.F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324 (1990).
Deming, D.A. et al. PIK3CA and APC mutations are synergistic in the development of intestinal cancers. Oncogene 33, 2245–2254 (2014).
Hinoi, T. et al. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 67, 9721–9730 (2007).
Martin, E.S. et al. Development of a colon cancer GEMM-derived orthotopic transplant model for drug discovery and validation. Clin. Cancer Res. 19, 2929–2940 (2013).
In, J.G. et al. Human mini-guts: new insights into intestinal physiology and host-pathogen interactions. Nat. Rev. Gastroenterol. Hepatol. 13, 633–642 (2016).
Roper, J. et al. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat. Biotechnol. 35, 569–576 (2017).
Qin, L., Liu, Y., Wang, J., Li, S. & Sato, Y. Neural and behavioral discrimination of sound duration by cats. J. Neurosci. 29, 15650–15659 (2009).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772 (2011).
Blokzijl, F. et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 538, 260–264 (2016).
Koo, B.K. et al. Controlled gene expression in primary Lgr5 organoid cultures. Nat. Methods 9, 81–83 (2012).
van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Fujii, M. et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18, 827–838 (2016).
Calon, A. et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 22, 571–584 (2012).
Zomer, A. et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046–1057 (2015).
Schwitalla, S. et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152, 25–38 (2013).
Fumagalli, A. et al. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc. Natl. Acad. Sci. USA 114, E2357–E2364 (2017).
Li, X. et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat. Med. 20, 769–777 (2014).
Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521, 43–47 (2015).
Matano, M. et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 21, 256–262 (2015).
Ritsma, L. et al. Surgical implantation of an abdominal imaging window for intravital microscopy. Nat. Protoc. 8, 583–594 (2013).
Beerling, E. et al. Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis-enhancing stem cell capacity. Cell Rep. 14, 2281–2288 (2016).
Hung, K.E. et al. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc. Natl. Acad. Sci. USA 107, 1565–1570 (2010).
Shibata, H. et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 278, 120–123 (1997).
Zechmann, C.M. et al. Impact of stroma on the growth, microcirculation, and metabolism of experimental prostate tumors. Neoplasia 9, 57–67 (2007).
Fidler, I.J. Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer Metast. Rev. 10, 229–243 (1991).
Cespedes, M.V. et al. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am. J. Pathol. 170, 1077–1085 (2007).
Melo, F.S. et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680 (2017).
Enquist, I.B. et al. Lymph node-independent liver metastasis in a model of metastatic colorectal cancer. Nat. Commun. 5, 3530 (2014).
Yui, S. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18, 618–623 (2012).
O'Rourke, K.P. et al. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat. Biotechnol. 35, 577–582 (2017).
Chassaing, B., Aitken, J.D., Malleshappa, M. & Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 104, Unit 15.25 (2014).
Zigmond, E. et al. Utilization of murine colonoscopy for orthotopic implantation of colorectal cancer. PLoS One 6, e28858 (2011).
Roper, J. et al. Colonoscopy-based colorectal cancer modeling in mice with CRISPR-Cas9 genome editing and organoid transplantation. Nat. Protoc. (2017).
Byrne, A.T. et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 17, 254–268 (2017).
Broutier, L. et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724–1743 (2016).
Drost, J., Artegiani, B. & Clevers, H. The generation of organoids for studying Wnt signaling. Methods Mol. Biol. 1481, 141–159 (2016).
Koo, B.K. et al. Controlled gene expression in primary Lgr5 organoid cultures. Nat. Methods 9, 81–83 (2011).
Fujii, M., Matano, M., Nanki, K. & Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 10, 1474–1485 (2015).
Fu, X.Y., Besterman, J.M., Monosov, A. & Hoffman, R.M. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc. Natl. Acad. Sci. USA 88, 9345–9349 (1991).
Klaver, Y.L., Lemmens, V.E., Nienhuijs, S.W., Luyer, M.D. & de Hingh, I.H. Peritoneal carcinomatosis of colorectal origin: incidence, prognosis and treatment options. World J. Gastroenterol. 18, 5489–5494 (2012).
Drost, J. et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 11, 347–358 (2016).
Chudakov, D.M., Lukyanov, S. & Lukyanov, K.A. Using photoactivatable fluorescent protein Dendra2 to track protein movement. Biotechniques 42 553, 555, 557 passim (2007).
Acknowledgements
We thank A. de Graaff and the Hubrecht Imaging Centre for imaging support. We thank O. Sansom and E. Hong Tan (Beatson Institute, Glasgow, UK) for providing the murine tumor organoid line. This work was financially supported by a Dutch Cancer Society Fellowship (BUIT-2013-5847 to S.J.E.S.), by the Dutch Cancer Society (KWF)/Alpe d′HuZes Bas Mulder Award (KWF/Alpe d′HuZes 10218, to J.D.), by European Research Council Grant CANCER-RECURRENCE 648804 (to J.v.R.), by the CancerGenomics.nl (Netherlands Organisation for Scientific Research) program (to J.v.R.), by the Doctor Josef Steiner Foundation (to J.v.R) and by the European Union′s Horizon 2020 research and innovation program under Marie Sklodowska-Curie grant agreement no. 642866 (to J.v.R).
Author information
Authors and Affiliations
Contributions
A.F. developed the orthotopic transplantation technique; A.F. and J.D. performed the experiments; H.B., E.B. and S.J.E.S. helped with data analysis and preparation of the figures; S.J.E.S edited the video; K.C.O. and H.J.S. provided the patient-derived CRC organoids; J.D. and J.v.R. supervised the study; A.F., J.D. and J.v.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
41596_2018_BFnprot2017137_MOESM136_ESM.mp4
Set up and demonstration of the surgical procedure to transplant CRC organoids into the caecal wall of recipient mice. (MP4 19876 kb)
Rights and permissions
About this article
Cite this article
Fumagalli, A., Suijkerbuijk, S., Begthel, H. et al. A surgical orthotopic organoid transplantation approach in mice to visualize and study colorectal cancer progression. Nat Protoc 13, 235–247 (2018). https://doi.org/10.1038/nprot.2017.137
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.137
This article is cited by
-
Sleeping Beauty transposon mutagenesis in mouse intestinal organoids identifies genes involved in tumor progression and metastasis
Cancer Gene Therapy (2024)
-
Intravital imaging of immune responses in intestinal inflammation
Inflammation and Regeneration (2023)
-
Lentiviral in situ targeting of stem cells in unperturbed intestinal epithelium
BMC Biology (2023)
-
Liver metastasis from colorectal cancer: pathogenetic development, immune landscape of the tumour microenvironment and therapeutic approaches
Journal of Experimental & Clinical Cancer Research (2023)
-
Degradation of HIF-1α induced by curcumol blocks glutaminolysis and inhibits epithelial-mesenchymal transition and invasion in colorectal cancer cells
Cell Biology and Toxicology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.