Abstract
Our understanding of endocytic pathway dynamics is restricted by the diffraction limit of light microscopy. Although super-resolution techniques can overcome this issue, highly crowded cellular environments, such as nerve terminals, can also dramatically limit the tracking of multiple endocytic vesicles such as synaptic vesicles (SVs), which in turn restricts the analytical dissection of their discrete diffusional and transport states. We recently introduced a pulse–chase technique for subdiffractional tracking of internalized molecules (sdTIM) that allows the visualization of fluorescently tagged molecules trapped in individual signaling endosomes and SVs in presynapses or axons with 30- to 50-nm localization precision. We originally developed this approach for tracking single molecules of botulinum neurotoxin type A, which undergoes activity-dependent internalization and retrograde transport in autophagosomes. This method was then adapted to localize the signaling endosomes containing cholera toxin subunit-B that undergo retrograde transport in axons and to track SVs in the crowded environment of hippocampal presynapses. We describe (i) the construction of a custom-made microfluidic device that enables control over neuronal orientation; (ii) the 3D printing of a perfusion system for sdTIM experiments performed on glass-bottom dishes; (iii) the dissection, culturing and transfection of hippocampal neurons in microfluidic devices; and (iv) guidance on how to perform the pulse–chase experiments and data analysis. In addition, we describe the use of single-molecule-tracking analytical tools to reveal the average and the heterogeneous single-molecule mobility behaviors. We also discuss alternative reagents and equipment that can, in principle, be used for sdTIM experiments and describe how to adapt sdTIM to image nanocluster formation and/or tubulation in early endosomes during sorting events. The procedures described in this protocol take ∼1 week.
Similar content being viewed by others
Introduction
Development of the sdTIM technique
Although the resolution of conventional optical microscopy is diffraction-limited, super-resolution microscopy techniques are able to accurately localize light-emitting molecules with higher accuracy. The development of super-resolution imaging methodologies has provided critical information on neuronal endocytic pathways, increasing our understanding of synaptic vesicle (SV) recycling1,2,3,4, synaptic activity5, neuronal survival6 and homeostasis7. Other studies have highlighted a large heterogeneity in SV population composition, dynamics and release properties within individual presynapses8,9,10,11,12,13,14, and, therefore, describing SV recycling with bulk measurements provides only limited insight into the SV recycling. One of the main limitations of the current super-resolution technologies available for direct visualization of SVs in presynapses is their limited ability to track multiple SVs simultaneously and to obtain a large number of trajectories to dissect discrete diffusional and transport states of the endocytic compartments. To address this, we developed the sdTIM method (Fig. 1), which allows the acquisition of thousands of relatively long single-molecule trajectories with high temporal resolution. This in turn allows dissection of the hidden mobility parameters, such as distinct diffusional and transport states, by applying hidden Markov models (HMMs) and Bayesian model selection15,16.
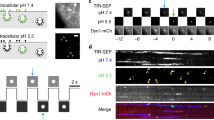
(a) Schematic representation of single fluorescent molecule detection in axons and nerve terminals using sdTIM under oblique illumination microscopy. (b) Hippocampal neurons grown in microfluidic devices (Steps 105–108) expressing VAMP2–pHluorin (Steps 109–114) are stimulated for 5 min with high-K+ buffer in the presence of anti-GFP nanobody (red) and CTB (blue) in the nerve terminal chamber and with high-K+ buffer in the soma chamber (pulse) (alternative Steps 117A and 117B). (c–e) After stimulation, the unbound nanobodies and CTB are washed off (c), and the neurons are chased for either 10 min (117A(vii); d) or 2 h in low-K+ buffer (Step 117B(x); e). (d) To detect internalized nanobodies inside individual SVs, the nerve terminals can be briefly exposed to laser illumination to bleach some of the fluorophores (optional). A subset of internalized nanobodies in SVs (red) can then be detected (blue box indicates imaging site within the terminal chamber) (alternative Steps 117A(viii) and 117B(x,xi)), thereby enabling single-molecule tracking (Steps 118–124). (e) After a 2-h chase (Step 117B(xi)), the retrograde transport of CTB and nanobodies can be imaged (yellow box indicates imaging site at the microfluidic channel). (f) Wide-field mosaic image of a hippocampal neuron expressing VAMP2-pHluorin and grown on a microfluidic device. The somatodendritic area of the neuron is on the right, and the axon passes through the microfluidic channel to the nerve terminal chamber. The boxed area at the bottom shows a bright-field image of the microfluidic channel, through which the axon (dashed-line box) passes. The directions of retrograde and anterograde transport are indicated in the figure. Scale bar, 50 μm. (Left inset) Photograph of a custom-made microfluidic device (Steps 1–53) used for polarization of neurons and compartmentalization of nerve terminals (terminal: blue) from the somatodendritic area (soma: red). Scale bar, 1 cm. (Right inset) The boxed area from the left inset is magnified, showing the microfluidic channels spanning the distance from the soma to the terminal well. Scale bar, 100 μm. All the experiments were carried out in accordance with relevant institutional and governmental ethical guidelines and regulations (Animal Ethics Approval QBI/254/16/NHMRC).
We first used sdTIM to investigate the role of presynaptic activity in the retrograde (toward the cell soma) transport of autophagosomes in live neurons7. Externally applied botulinum neurotoxin type A (BoNT/A) is internalized in an activity-dependent manner into SVs and endosomes17 and reaches autophagosomes that are retrogradely transported back to the cell body7. We also used sdTIM to study the retrograde flux of signaling endosomes initiated upon presynaptic activity by localizing Alexa Fluor 647-conjugated cholera toxin subunit-B (Alexa647-CTB) in axons6. We discovered that synaptic activity controls the retrograde transport of autophagosomes7, as well as that of signaling endosomes6,7, to the soma. Most recently, we adapted this method to study the mobility of individual recycling SVs in presynapses by overexpressing pHluorin-tagged vesicle-associated membrane protein 2 (VAMP2-pHluorin) in hippocampal neurons18. This adaptation was based on activity-dependent internalization of externally applied anti-GFP Atto-647N-labeled nanobodies (Atto647N-NBs), which bind specifically to pHluorin (the pH-sensitive version of GFP)18. We revealed that SVs constantly switched between a low (immobile) diffusional state and two transport states of opposite direction. In the current protocol, we have extended our original pulse–chase method6,7,18 for sequential dual-color super-resolution imaging of endocytic structures. Using VAMP2-pHluorin-bound anti-GFP Atto565-nanobodies (Atto565-NBs) in conjunction with Alexa647-CTB, we demonstrate that simply by choosing an appropriate fluorescent ligand and by adjusting the time course of the pulse chase, sdTIM can be adapted to dual-color imaging of multiple endocytic compartments.
Overview of the procedures
In this 1-week protocol, we describe the sdTIM pulse–chase method, which is based on activity-dependent internalization of fluorescent ligands into subdiffractional endocytic compartments, such as SVs and signaling endosomes, which can then be visualized in a crowded presynaptic environment or at adjacent axons. The protocol consists of 125 steps. We start by describing the fabrication of custom-made microfluidic devices (Steps 1–53) for polarized culturing of hippocampal neurons (Fig. 1), which allows the reliable identification of presynapses and axons and the unequivocal identification of the long-range retrograde (toward the soma) and anterograde (toward nerve terminals) axonal transport. It also allows localized manipulation of specific neuronal regions such as the nerve terminals and soma. We then describe the construction of a custom-made 3D-printed perfusion system (Steps 54–66; Supplementary Fig. 1) that allows the sdTIM technique to be performed without the need of removing the cell culture dish from the microscope between the protocol steps. The perfusion system permits efficient liquid exchange in the culture dish without excessive disturbance of the neurons, and, more importantly, it enables the identification of mature and active presynapses, and, hence, a targeted analysis of these distinct neuronal regions. We then describe an important control step of analyzing the fluorescently labeled ligands used for sdTIM experiments, which should be carried out before single-particle-tracking experiments (Steps 67–78).
The sdTIM pulse–chase protocol can be carried out in two cell culture plate formats: super-resolution-compatible glass-bottom dishes and microfluidic devices. We describe the dissection and plating procedure (Steps 79–105) and culturing (Steps 106–108) for hippocampal neurons in these two dish formats. Mature hippocampal neurons (Box 1) are transfected with VAMP2-pHluorin for 24–48 h (Steps 109–114) and then stimulated (pulse) with a high-K+ buffer (pulse) in the presence of Atto647N-NBs for 5 min to induce synaptic activity and the uptake of fluorescently labeled ligands (Steps 115–117A). For dual-color imaging, the stimulation is done with high-K+ buffer in the presence of Atto565-NBs and Alexa647-CTB for 5 min (Step 117B). Note that for efficient neuronal stimulation in microfluidic devices, both the soma and nerve terminal chambers are simultaneously stimulated with high-K+ buffer, whereas only the nerve terminal chamber is supplemented with fluorescent ligands. After stimulation, the neurons are washed with low-K+ buffer (Fig. 1c) to remove unbound Atto647N-NBs (Step 117A(vii)) or Atto565-NB and Alexa647-CTB (Step 117B(viii and ix)). The neurons are then incubated (chase) for either 10 min or 2 h, respectively, in low-K+ buffer (Fig. 1d,e). To detect internalized nanobodies inside individual SVs, the nerve terminals can be briefly exposed to laser illumination (optional), and the remaining Atto647N-NBs can be subsequently imaged under oblique (or inclined) illumination microscopy (Steps 117A(viii) or 117B(x–xii); Fig. 1d; Supplementary Video 1), thereby enabling single-molecule tracking of SVs in presynapses (Steps 118–124). Similarly, after the 2-h chase (Fig. 1e), CTB-containing signaling endosomes can be detected with oblique illumination microscopy as they undergo axonal retrograde transport and carry survival signals from presynapses to the soma (Step 117B(x)). Oblique illumination uses a slightly smaller angle than that of total internal reflection fluorescence (TIRF) microscopy, in which the sample is illuminated with an evanescent field that yields a thin optical section19,20,21. By decreasing the laser angle, oblique illumination increases the optical section thickness, which allows imaging beyond the basal surface of the cell. Although we do not provide complete details of the imaging aspect per se, as this is more likely to be specific to each sdTIM technique adaptation, we discuss the implementation of the method in other cellular settings (please see the Alternative equipment section). And finally, we describe how to quantify the number of internalized Atto647N-NBs in SVs to estimate the number of SVs that can be detected at any given time (Step 125).
Importantly, we also explain how to discriminate active presynapses from adjacent axons by monitoring the exocytosis associated with synaptic transmission using pHluorin, a pH-sensitive GFP22 (Box 1; Supplementary Fig. 2), and we describe how to assess synaptic identity, functionality and maturity (Box 1; Supplementary Fig. 3), all of which are critical controls for the sdTIM technique. We also discuss the appropriate use of fluorescent probes for sdTIM super-resolution experiments (Box 2) and describe steps for single molecule tracking with freely available software (Supplementary Fig. 4).
Applications of the method
We originally used sdTIM to image retrograde transport of single molecules of BoNT/A in autophagosomes7. Although autophagosomes are large structures that can be imaged with conventional light microscopy, a very low pathological concentration of BoNT/A23 can be detected only with super-resolution microscopy, as only a few molecules are likely to enter each retrograde carrier. Therefore, the sdTIM method allows imaging of the autophagosome initiation process or immobilization of proteins in nanodomains of the autophagosomes. We have also used sdTIM to track nanobodies internalized in SVs in presynapses18 using oblique illumination. An adaptation of this method was also used for localizing retrogradely transported CTB in signaling endosomes using structured illumination microscopy (SIM)6. In the studies in which we have applied sdTIM, we have used primary hippocampal neurons from rats6,7,18. SV trafficking is affected in many neurodegenerative conditions, including Alzheimer's and Parkinson's disease24, and studying how key inter- and intramolecular interactions affect SV mobility in appropriate disease mouse models may, ultimately, provide new insights into the associated disease pathogenesis. In addition, we anticipate that the sdTIM method could potentially be adapted to any other endocytic cell systems; it is directly applicable to the detailed analysis of the location and mobility of various presynaptic molecules undergoing activity-dependent internalization, recycling and/or retrograde/anterograde transport in the context of genetic or pharmacological manipulations. Finally, sdTIM could potentially be applied to simultaneous dual-color imaging by using an appropriate imaging system to image the mobility of two fluorophores at the same time. As nanobodies with a variety of fluorescent tags displaying specificity for different fluorophores are commercially available, dual-color imaging would greatly expand the applicability of sdTIM.
The use of microfluidic devices will also allow the study of polarized cell–cell interactions. For example, synaptic connections could be studied by transfecting the microfluidic soma and terminal wells with pre- and postsynaptic markers, respectively. Other cell–cell connections, such as neuromuscular junctions, could be studied by culturing muscle cells in conjunction with neurons in the microfluidic chambers. In addition, microfluidic devices allow a more region-specific application of pharmacological agents to the cell culture dish. The use of oblique illumination allows imaging in deeper regions of the cell than with TIRF microscopy.
Comparison with other methods
Several single-molecule techniques have been introduced to characterize the mobility of SVs1,3,25,26,27 and the retrograde transport of endosomes in axons28,29. The majority of the current single-particle-tracking (spt) techniques, such as those using photoconvertible or pH-sensitive fusion proteins, or styryl dyes, generate relatively low numbers of short trajectories1,2,3,30,31,32. The obtained trajectory lengths depend on several aspects, such as laser power and imaging rate, the choice of fluorophore and the imaging conditions in general. Using Atto647N-NBs, the average length of the SV trajectories in presynapses in our experiments was 0.4 ± 0.04 s (n = 32,000 trajectories from presynaptic regions of 12 hippocampal neurons in independent cultures) with an imaging rate of 50 frames/s. Longer trajectories (up to 50 s) have been obtained using quantum dot (Qdot)-based single-molecule imaging with an imaging rate of 10 frames/s29. However, Qdot probes suffer from a number of drawbacks, one of them being their relatively large size (5–25 nm)33, which could affect their internalization. By contrast, the sdTIM technique relies on the activity-dependent internalization of either CTB or VAMP2-pHluorin-bound small anti-GFP nanobodies conjugated with photostable fluorophores. This ensures minimal interference with the endocytic transport machinery while creating a large number of relatively long trajectories. sdTIM is a fast and simple pulse–chase-based technique that takes advantage of several technological advancements to produce high-density data (several thousand trajectories) at a 50-Hz imaging rate18 and with high localization precision (43 ± 9 nm for CTB in signaling endosomes, 36 ± 1 nm for Atto647N-NBs18 and 32 ± 4 nm for Atto565-NBs in SVs) (Supplementary Fig. 5c,d). The SV pools within individual synapses exhibit a vast heterogeneity in terms of composition and release properties9,10,11,12,13. Such intrinsic heterogeneity underscores the limitations of using bulk measurements and averaging to understand SV recycling. The main advantages of sdTIM are that it produces hundreds of trajectories for each presynapse and thousands for each neuron, enabling the identification of a wide range of diffusion coefficients that are otherwise lost in the commonly used process of time averaging. This is ideal for tracking multiple SVs over long periods of time using single-particle nanoscopy. The advantage of using custom-made microfluidic devices is that they allow culturing of polarized hippocampal neurons, which assists with the discrimination of morphological features and unequivocal identification of nerve terminals and axons. On the other hand, the use of the microfluidic chambers requires experience in both the microfabrication of the chambers and the seeding, maintenance and manipulation (e.g., transfection, adding of reagents) of the neurons.
Limitations
The SV adaptation of the sdTIM technique relies on the overexpression of a pHluorin- (or GFP-) tagged marker destined to cycle between the plasma membrane and SVs in an activity-dependent manner. Consequently, the pHluorin tag must be exposed to the extracellular space to be available for externally applied anti-GFP nanobody binding. Therefore, in this setting, sdTIM is restricted to imaging SVs from the recycling pool. However, increasing the strength and/or duration of the pulse should allow investigation of the reserve pool of SVs. This limitation could also be addressed by using dual-color fluorescence imaging of two different ligands (Fig. 2; Supplementary Table 1). Moreover, because of the natural cycling of VAMP2 between the plasma membrane and SVs34,35, we cannot exclude the possibility that a small portion of single-molecule detections originate from the plasma membrane (or structures other than SVs). This could be monitored with electron microscopy (EM) by studying the internalization of VAMP2-pHluorin-bound HRP-tagged nanobodies and estimating the proportion of internalized nanobodies in SVs18 (Box 1; Supplementary Fig. 3c). Furthermore, the diffusion coefficient values could be used as a filter to discriminate plasma-membrane-resident probes from those that are internalized in SVs18,36.
In addition to using sdTIM to image a variety of SV proteins, the method can be applied to track any labeled cargo molecules along different endocytic pathways. Alternatively, sdTIM can be used for imaging nanoscale immobilization in large early endosomal structures. Combining sdTIM with sptPALM for imaging fluorescently labeled endocytic cargo in conjunction with intra- or extracellular overexpressed mEos-tagged (or analog) proteins, respectively, can be used. AB, antibody; AP1, clathrin adaptor protein 1; BoNT/A, botulinum neurotoxin type A; BoNT/A-LC, botulinum neurotoxin type A light chain; CI-MPR, cation-independent mannose 6-phosphate receptor; CLIC, clathrin-independent carrier; COG3, component of oligomeric Golgi complex 3; EGFR, epidermal growth factor receptor; ER, endoplasmic reticulum; FEME, fast endophilin-mediated endocytosis; GEEC, glycosylphosphatidylinositol (GPI)-enriched endocytic compartments; GRAF1, GTPase regulator associated with focal adhesion kinase; ILR2, interleukin-2 receptor; PACS1, phosphofurin acidic cluster sorting protein 1; SNX, sortin nexin; SV, synaptic vesicle; SV2, synaptic vesicle protein 2; TeNT, tetanus neurotoxin; TfnR, transferrin receptor; VAMP, vesicle-associated membrane protein; VGLUT1, vesicular glutamate transporter 1; VTI1A, vesicle transport through interaction with t-SNAREs 1A. Please see Supplementary Table 1 for further details. Image adapted with permission from ref. 61, Elsevier.
The application of sdTIM to study SV mobility in presynapses relies on the overexpression of a GFP-tagged protein of interest to allow nanobody binding. Consequently, the expression level of the GFP-fusion construct must be tightly controlled and verified when studying single-molecule mobility. Such overexpression is not required when studying the retrograde transport of CTB or BoNT/A, which makes these approaches less invasive, as they instead rely on the tracking of ligands that bind to endogenous proteins. Styryl dyes can be used only to monitor general endocytosis, whereas expressing VAMP2-pHluorin does provide sufficient specificity to study nanobody internalization into SVs. In our system, VAMP2-pHluorin-bound Atto647N-NBs display a mobility18 similar to that reported earlier for other major SV proteins27. One of the disadvantages of sdTIM is that genetic manipulation of an exocytic protein to block exocytosis would also prevent endocytosis of the single-molecule probes. The use of spt–photoactivated localization microscopy (sptPALM)30 with a photoconvertible tag of vesicular markers would therefore be more appropriate.
Another disadvantage of sdTIM is that it requires oblique illumination and is therefore limited to imaging in a thin optical section adjacent to the glass bottom of the culture dish. This can be particularly challenging when using microfluidic devices to study the retrograde transport of CTB, as it is difficult to find long continuous stretches of the axon in the same imaging plane. Similar to the majority of other super-resolution techniques, such as universal point accumulation for imaging in nanoscale topography (uPAINT) and SIM, sdTIM is currently limited to 2D4,20. This limitation could potentially be overcome by using astigmatic lenses and/or commercial 3D visualization and analysis tools (e.g., SRX software for the Vutara 352 super-resolution microscope by Bruker)37. Recent studies have also extended the use of stimulated emission depletion (STED) and direct stochastic optical reconstruction microscopy (dSTORM) applications to 3D with ∼20- to 100-nm axial resolution3,38,39,40.
Experimental design
Sample size and controls. To obtain meaningful data, we recommend using a minimum of 12–20 hippocampal neurons per condition. These 12–20 neurons should originate from at least three neuronal cultures from independent dissections. Note that only one neuron per plate can be imaged for an sdTIM experiment. Until you become more experienced with the protocol, we recommend preparing a few additional plates per condition, on the assumption that some of the experiments will not be optimal (in regard to, e.g., transfection efficiency, neuron confluence and proportion of microglia versus neurons on the plate). In a typical experiment, we plate hippocampal neurons on six plates (three for controls and three for treatment), which will yield, on average, 8,000 SV trajectories per neuron when using Atto647N-NBs18. On average, 250 trajectories can be obtained from an individual presynapse or an axonal segment18.
To ensure unequivocal discrimination of active presynapses from adjacent axons, we recommend monitoring the exocytosis associated with synaptic transmission using pH-sensitive GFP22 (Box 1; Supplementary Fig. 2). Other critical controls for the sdTIM technique are the assessment of synaptic identity, functionality and maturity (Box 1; Supplementary Fig. 3). We also recommend that the researcher quantify the fluorophore-labeling density of the ligands (Fig. 3a) and estimate the number of internalized ligands in an endocytic compartment (Fig. 3b). The appropriate use of fluorescent probes for sdTIM super-resolution experiments must be taken into account (Box 2).
(a) Highly diluted Atto647N-NBs were deposited on a glass surface at pH 7.4. The graph shows a representative intensity–time (s) trace of the time recording of Atto647N-NB fluorescence from 5 × 5 pixels around the center of the fluorescent spot. The emission trace shows an example of one-step fluorescence emission. Representative fluorescence image captures of the Atto647N-NB are shown before excitation (i), during excitation (ii) and after bleaching (iii) corresponding to the time trace, and the red square represents the region of interest. (b) To estimate the number of internalized fluorophores per SV, hippocampal neurons expressing VAMP2-pHluorin were subjected to sdTIM and fixed. A representative two-step intensity–time trace (s) of an Atto647-NB obtained from a fixed presynapse is shown. Dotted red lines indicate the detected emission steps. All the experiments were carried out in accordance with relevant institutional and governmental ethical guidelines and regulations (Animal Ethics Approval QBI/254/16/NHMRC).
Alternative equipment. Any microscope with a suitable single-molecule-detection speed (here 20 ms) can be used to implement this technique (note that the requirement for the detection speed depends on the time scale of the biological phenomenon). However, the methodology we describe here could be adapted to other microscopy platforms, which, as a consequence, broadly expands its potential applicability. This protocol can be used with the recently developed spinning disk super-resolution microscope (SDSRM)41 currently available from Olympus and Roper Scientific, in the spinning disk optical photon reassignment scheme (SD-OPR)42 soon to be available from Yokogawa, or with instant SIM (iSIM)43, currently available from VisiTech International. Super-resolution by radial-fluctuation (SRRF) analysis44 could also be used with any wide-field, confocal or TIRF microscope, such as the Andor Dragonfly, with which it has already been implemented for real-time processing. Recently, an extended-resolution SIM platform has been developed45, with a lateral resolution <100 nm, but unfortunately the acquisition speed is at least one order of magnitude lower than that of the aforementioned methods. These systems achieve lower spatial resolution (up to 120–150 nm) than our method, but most of them enable very high acquisition speeds (up to 100 Hz). The high speed and low phototoxicity make them perfect options for live-cell imaging. The mentioned resolution could be a problem when imaging very small and crowded structures such as vesicles in synaptic terminals; however, this would not be any problem in the study of other endocytic processes (Fig. 2). In Figure 2, we suggest different cargoes (TfnR, viruses, BoNT/A, TeNT, cholera toxin, EGFR, drug delivery antibody and a penetrating peptide) that, if appropriately tagged with super-resolution fluorophores, could potentially be used to track virtually all the described types of endocytosis. As a consequence, small modifications of the new methodology described in our paper could be made using a large variety of microscopes, potentially expanding the study of endocytic processes of cells and achieving a lateral resolution that exceeds the classic diffraction limit by a factor of more than two.
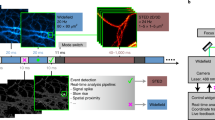
Considerations for single-molecule tracking. Many software packages are available for single-molecule detection and tracking46. We use a combination of wavelet segmentation47 and optimization of multiframe object correspondence by simulated annealing48, using a PALM-Tracer49,50 plug-in for the MetaMorph software for single-molecule tracking (Figs. 4 and 5). Importantly, we also show the expected results regarding the heterogeneous nature of the mobility states displayed by internalized single molecules (Fig. 6; Supplementary Fig. 5). These highlight the capacity of sdTIM to dissect the hidden mobility states of subdiffractional endocytic structures and to reveal unprecedented details of the heterogeneous nature of their mobility. Alternatively, freely available software such as TrackMate51 can be used for single-molecule tracking. However, it is worth noting that when using such software for single-molecule tracking, it may be necessary to develop additional analysis routines in programming languages such as MATLAB (MathWorks, https://www.mathworks.com/) or Python (https://www.python.org/) in order to obtain single-molecule-mobility parameters. We describe the steps for single-molecule tracking with TrackMate and provide a MATLAB routine that calculates mean-square displacement curves and diffusion coefficients51 (Supplementary Fig. 4; Supplementary Data 1, four files in total are included).
(a) sdTIM experimental time line. Hippocampal neurons grown on glass-bottom dishes and transfected with VAMP2-pHluorin after DIV14–16 are stimulated in anti-GFP Atto647N-NB (3.2 pg μl−1) containing high-K+ buffer for 5 min (pulse), then chased for 10 min in low-K+ buffer. After the chase, single-molecule imaging of internalized Atto647N-NB in SVs is performed under oblique illumination microscopy. (b) Wide-field fluorescence image of a high-K+-stimulated hippocampal neuron expressing VAMP2-pHluorin superimposed on a bright-field image of hippocampal neurons grown on a glass-bottom cell culture dish (nerve terminal chamber of the microfluidic device). (c) The corresponding super-resolved sptPALM intensity map of VAMP2-phluorin-bound Atto647N-NB localizations from 8,000 trajectories. The colored bar represents localization densities, for which the color coding of the localization densities is shown as arbitrary units, the warmer colors indicating higher density. (d) sptPALM diffusion coefficient map of Atto647N-NB localizations from 8,000 trajectories. The color scale range from –4 to 0 corresponds to Log10 diffusion coefficient detections (μm2s−1), and the warmer colors in the scale indicate lower mobility. (e) Corresponding trajectories of Atto647N-NB mobility over 16,000 frames. Trajectory color coding corresponds to acquisition frame number. Boxed area in b is shown at a higher magnification in the bottom corner. Corresponding areas are indicated in c–e. Scale bars, 10 μm (b–e); 1 μm (insets). (f–i) Quantification of the SV mobility in presynapses (Ps; n = 5) and axonal segments (Ax; n = 11) of the neuron shown in (b) are shown. The graphs show the frequency distribution of the average Log10 diffusion coefficient (Log10D) (f), mobile-to-immobile (M/IMM) ratios (g), the mean square displacement (MSD (μm2)) (h), and the area under the MSD curve (AUC (μm2 s)) of VAMP2–pHluorin-bound Atto647N-nanobodies in presynapses and axons (i). The average numbers ±SEM presented in f–i are derived from the 5 presynapses (1,300 trajectories) and 11 axonal segments (2,100 trajectories) of the neuron presented in b–e, and the dots in g and i represent measurements from individual presynapses and axonal segments. All the experiments were carried out in accordance with relevant institutional and governmental ethical guidelines and regulations (Animal Ethics Approval QBI/254/16/NHMRC).
(a) sdTIM experimental time line. Hippocampal neurons are stimulated in the presence of Alexa647-CTB- (50 ng ml−1) and Atto565-NB (3.2 pg μl−1)-containing high-K+ buffer for 5 min (pulse) and chased for 2 h in growth medium. After the chase, single-molecule imaging is performed under oblique illumination microscopy in the axon channel. (b) Wide-field image of a hippocampal neuron expressing VAMP2-pHluorin superimposed on a bright-field image of the microfluidic device channel. The image is taken from the soma chamber side of the channel. (c,d) Time-lapse (s) images of the retrograde transport of Alexa647-CTB (open arrowheads) and Atto565-NBs (filled arrowheads; c) from the boxed region in b, and selected Alexa647-CTB (green) and Atto565-NBs (magenta) trajectories (d) from the dashed line box in b. The inset in d shows an overlay of the trajectories and the bright-field image of the axon channel. Representative traces for Alexa647-CTB (open arrowhead) and Atto565-NBs (filled arrowhead) are magnified in e. Scale bars, 5 μm (b,d (main and inset),e) and 1 μm (c); scale bar in b applies to inset in d. (f–i) Mobility parameters of the CTB carriers (n = 17 hippocampal neurons in 3 individual cultures) and SVs (n = 9 hippocampal neurons in 3 individual cultures) in the neurons. The graphs show (f) frequency distribution of the Log10D, (g) cumulative frequency of Log10D, (h) MSD (μm2) and (i) AUC (μm2 s) of Alexa647-CTB and VAMP2–pHluorin-bound Atto565-NBs, respectively. The average numbers ±SEM of CTB carriers (2,600 trajectories) and SVs (5,500 trajectories) presented in f, h and i are derived from the axons passing through microfluidic channels, and the dots in i represent measurements from individual neurons. All the experiments were carried out in accordance with relevant institutional and governmental ethical guidelines and regulations (Animal Ethics Approval QBI/254/16/NHMRC).
(a) Representative trajectory of retrogradely transported Alexa647-CTB (Fig. 5e). (b) Kymograph of Alexa647-CTB highlights the active transport and stationary (asterisks) phases along the trajectory. (c) MSD (μm2) of the trajectory in a. The fit of equation MSD(τ) = 4Dτ + v2τ2 (line) to the MSD curve (circles), where v is the average velocity, D is the diffusion coefficient and τ is the time lag. (d) The same CTB trajectory shown in a displays pure diffusion (D, red) and transport (DV, blue) motion states inferred by HMM–Bayes analysis. The trajectory is annotated and color-coded with the respective motion states. The time line shows the temporal (s) sequence of the inferred D and DV motion states, and the boxed area is shown at higher magnification in the middle. (e) Red and blue circles represent the two detected motion states, and the circle area is proportional to the percentage occupancy in the respective state. The diffusion coefficients (D1 and D2) of the motion states and the velocity magnitude of the retrograde transport state (V2) are indicated. Localization precision of 30 nm (Supplementary Fig. 5) was used to correct the diffusion coefficients. Scale bars, 1 μm (unless otherwise stated). All the experiments were carried out in accordance with relevant institutional and governmental ethical guidelines and regulations (Animal Ethics Approval QBI/254/16/NHMRC).
Adaptations to follow the internalization of other SV proteins or different plasma membrane proteins destined for endocytosis. Here, we use VAMP2-pHluorin as an SV marker. To identify different populations of SVs, the pHluorin moiety could be tagged to the intravesicular domain of other SV proteins. Similarly, the fluorophore could be genetically conjugated with any plasma membrane protein (e.g., G-protein-coupled receptors, receptor tyrosine kinases and glycosylphosphatidylinositol (GPI)-anchored proteins) that, after endocytosis, is delivered into an acidic compartment (Fig. 2). As described above, quenching of pHluorin fluorescence indicates internalization of the labeled protein into the acidic lumen of the targeted endocytic vesicle or organelle. Control studies should be performed to ensure that the pHluorin conjugation does not change the function and/or localization of the target protein. This can, for example, be done by comparing the kinetics and/or localization of antibody-labeled endogenous SV proteins and pH-sensitive SV markers. As demonstrated previously26, the kinetics of SV recycling is not affected by VAMP2–pHluorin overexpression in neurons.
Adaptation to follow different endocytic cargoes. Here, we used CTB labeled with Atto647N as an endocytic cargo. In addition, similar fluorophores can be conjugated to other extracellular molecules whose function relies on endocytosis and intracellular membrane transport (Fig. 2). In many cases, the molecular details of how a given exogenous molecule interacts with the cell are largely missing. Examples include hormones such as brain-derived neurotrophic factor (BDNF), glial-cell-derived neurotrophic factor (GDNF), receptor orphans, cerebral dopamine neurotrophic factor (CDNF) and mesencephalic, astrocyte-derived neurotrophic factor (MANF)52,53,54. Other examples include monoclonal antibodies and cell-penetrating peptides used for drug delivery55,56,57, and microbes, including viruses58,59,60,61 and bacteria62. The size, physiological abundance and dynamics of each of these cargoes can be very different. Hence, the user will have to adjust the key parameters, such as concentration of the cargo, labeling conditions (i.e., the number of fluorophores per cargo molecule), pulse and chase times, imaging rate and total length of acquisition.
Adaptation to study cellular endocytic and membrane-trafficking regulators. Knowledge of which set of cellular proteins regulates the uptake and intracellular transport of a given cargo molecule is key to understanding its function. Hundreds of cytosolic proteins regulate membrane dynamics at the plasma membrane and affect intracellular membrane trafficking, including that of endosomes63,64,65,66,67,68. These include Rab- and Rho-GTPases, membrane-bending proteins (proteins containing BAR domains), lipid-modifying enzymes and cytoskeletal components. In principle, each of these proteins could be genetically coupled to mEos, a photoconvertible fluorescent protein, or analogous fluorescent proteins, and could be imaged simultaneously with Atto-labeled endocytic cargoes69 (Fig. 2). The recent development of gene-editing tools such as the CRISPR technology70 allows knockout or knock-in of any protein tagged with mEos. With this modification, our sdTIM method becomes particularly useful in the study of the dynamics of diffraction-limited endosomal membrane nanodomains, in which sorting of endocytic cargoes and their receptors occurs71,72,73. This 'mosaic' of nanodomains at the cytosolic side of early/maturing endosomes is generated by the interplay of cargo/receptor complexes and a set of cytosolic proteins (e.g., phosphoinositide kinases, phosphatases and sorting nexins) that coordinate membrane tubulation, recruitment of actin-nucleating enzymes and other proteins, and ultimately induce the formation of sorting-vesicle carriers74,75,76,77. Control studies should be performed to ensure that the mEos tag does not disturb the function and localization of the labeled protein.
Adaptation for virus–cell interaction studies. The approach outlined above can be further adapted to study the early steps of virus infections. After binding to their cognate receptors at the plasma membrane, most viruses are internalized via endocytosis and transported to different intracellular compartments, where they replicate. During the past decade, a plethora of cellular proteins that are required for (or restrict) viral infections have been identified78. Many of these proteins regulate the penetration steps of the virus and their mode of action awaits characterization. To be imaged by sdTIM, virus particles can be labeled with fluorophores such as Atto647N79,80 and the cellular protein of interest can be conjugated with mEos or a photoconvertible analog (Fig. 2). Most of the studies in which super-resolution microscopy has been used to image viruses have been performed on fixed cells81,82,83, and only a few of these techniques have been applied to live cells84. Our method is particularly suitable for live-cell imaging studies. In contrast to toxins and single proteins, however, viruses are large macromolecular particles with sizes (i.e., diameters) that can be similar (e.g., poliovirus: ∼30 nm), larger (e.g., HIV, Zika and rabies virus: 40–180 nm) or much larger (e.g., vaccinia virus: ∼270 × 250 × 360 nm) than the localization precision of the sdTIM. This should be taken into account when interpreting the results of the image analysis, particularly for colocalization studies.
Materials
REAGENTS
The microfabrication of the microfluidic device
-
Microfluidic mask and master design: see Supplementary Data 2 for the creation of the mask and the master, and for the fabrication of the microfluidic device
-
AZ 726MIF Developer (Microchemical, cat. no. AZ726 MIF)
Caution
AZ 726MIF developer is a tetramethylammonium hydroxide (TMAH)-based developer that is corrosive; it causes burns upon exposure to the skin. Use it only in a chemical fume hood with proper personal protective equipment.
-
Chromium etchant (Transene, Chromium Etchant 1020)
Caution
Chromium etchant is a nitric acid- and ceric ammonium-nitrate-based etchant that is corrosive; it causes burns by all exposure routes. Use it only in a chemical fume hood with proper personal protective equipment.
-
Acetone (VLSI-grade; Sigma-Aldrich, cat. no. 40289)
Caution
Acetone is flammable and it may cause skin and eye irritation. Avoid contact with the skin, eyes and clothing, and handle it with gloves in a chemical fume hood.
-
Isopropanol (VLSI-grade; Sigma-Aldrich, cat. no. 40301)
Caution
Isopropanol is flammable and it may cause skin and eye irritation. Avoid contact with the skin, eyes and clothing, and handle it with gloves in a chemical fume hood.
-
Sulfuric acid, 95–98% (wt/vol) (reagent-grade; Sigma-Aldrich, cat. no. 258105)
Caution
Concentrated sulfuric acid is a strong acid and an oxidizer; it causes burns by all exposure routes. Use it only in a chemical fume hood with proper personal protective equipment.
-
Hydrogen peroxide, 30% (vol/vol) (reagent-grade; Sigma-Aldrich, cat. no. 216763)
Caution
Concentrated hydrogen peroxide is a corrosive oxidizing agent and may cause skin and eye irritation or burns. Use it only in a chemical fume hood with proper personal protective equipment.
-
SU8-2005 (Microchemical, cat. no. SU8-2005)
Caution
SU8-2005 is flammable and may cause severe skin and eye irritation. Avoid contact with the skin, eyes and clothing, and handle it with gloves in a chemical fume hood.
-
SU8-2100 (Microchemical, cat. no. SU8-2100)
Caution
SU8-2100 is flammable and may cause severe skin and eye irritation. Avoid contact with the skin, eyes and clothing, and handle it with gloves in a chemical fume hood.
-
SU8-2000 thinner (Microchemical, cat. no. SU8 Thinner)
Caution
SU8-2000 Thinner is flammable and may cause severe skin and eye irritation. Avoid contact with the skin, eyes and clothing, and handle it with gloves in a chemical fume hood.
-
Propylene glycol monomethyl ether acetate (reagent grade; Sigma-Aldrich, cat. no. 484431)
Caution
Propylene glycol monomethyl ether acetate is flammable and may cause severe skin and eye irritation. Avoid contact with the skin, eyes and clothing, and handle it with gloves in a chemical fume hood.
-
Trichloro(1H,1H,2H,2H-perfluorooctyl)silane (Sigma-Aldrich, cat. no. 448931)
Caution
Trichloro(1H,1H,2H,2H-perfluorooctyl)silane is corrosive and causes burns by all exposure routes, especially through inhalation. It can react violently with water, and it can produce highly toxic gases under fire conditions. Use it only in a chemical fume hood with proper personal protective equipment.
-
SYLGARD 182 silicone elastomer kit, polydimethylsiloxane (PDMS; Dow Corning, cat. no. 1064282)
-
Propylene glycol monomethyl ether acetate (PGMEA; Sigma-Aldrich, cat. no. 484431)
-
Trichloro(1H,1H,2H,2H-perfluorooctyl)silane (fluorosilane; Sigma-Aldrich, cat. no. 448931)
-
PBS (Thermo Fisher Scientific, cat. no. 10010-023)
-
70% (vol/vol) Ethanol (generic; UQ Stores, cat. no. US004284)
-
Milli-Q water (EDM Millipore, cat no. Z00Q0V0WW)
-
35-mm Glass-bottom cell culture dishes (Cellvis, cat. no. D35-20-1.5N or equivalent)
3D printing of custom-made perfusion lid and preparation of perfusion chamber
-
ABS-like resin (3DMaterials, cat. no. SKU001)
-
Absolute ethanol (>98% pure; Merck, cat. no. 100983 or equivalent)
-
Tap water
Assessment of the fluorescent labeling of ligands and the number of internalized ligands
-
Anti-GFP nanobodies (a gift from K. Aleksandrov, Institute for Molecular Bioscience, The University of Queensland, Australia) labeled with Atto647N or Atto565 as described earlier17,18
Critical
Alternatively, equivalent commercial camelid sdAB can be purchased, e.g., from Synaptic Systems, cat. no. N0301-At647N-S or N0301-At565-S.
Critical
Fluorescently labeled nanobodies are light-sensitive and should therefore be protected from light, e.g., by wrapping the stock tube and the prepared dilution in aluminum foil. Avoid repetitive freeze–thaw cycles by storing the nanobodies in 10- to 20-μl aliquots at −80 °C for up to 12 months. For best results, thaw the frozen aliquots on ice (protected from light) immediately before the experiment and always prepare fresh dilutions for each experiment.
-
Potassium hydroxide (Sigma-Aldrich, cat. no. V000105-VETEC)
-
Paraformaldehyde (PFA) 16% (wt/vol) (VWR, cat. no. 100503-914)
Caution
PFA is a tissue fixative. Handle it with appropriate protective clothing, gloves and goggles. Work in a fume hood and discard by following institutional regulations.
-
PBS, pH 7.4
Critical
For experiments in an acidic environment or at a pH value other than neutral, titrate the PBS with HCl to pH 5.0 or to an appropriate pH and then use it immediately.
-
Milli-Q water (Millipore)
Buffer preparation
-
HEPES (Sigma-Aldrich, cat. no. H3375)
-
Ascorbic acid (Sigma-Aldrich, cat. no. A5960)
-
CaCl2 (Sigma-Aldrich, cat. no. C5080)
-
BSA (Sigma-Aldrich, cat. no. A8022)
Caution
Wear appropriate eye and skin protection when using BSA.
-
NaCl (Amresco, cat. no. X190)
-
D-Glucose (Amresco, cat. no. 0188)
-
KCl (Ajax Finechem, cat. no. 1206119)
-
MgCl2 (Chem-Supply, cat. no. MA029)
-
HCl (Fluka, cat. no. 35328)
-
NaOH (Chem-Supply, cat. no. SA178)
Caution
NaOH may cause chemical burns, permanent injury or scarring, and blindness. Wear appropriate eye and skin protection.
-
NaHCO3 (Merck Millipore, cat. no. 144-55-8)
-
Milli-Q water (Millipore)
Preparation of the PLL coating
-
Poly-L-lysine hydrobromide (PLL), MW 30,000–70,000 (Sigma-Aldrich, cat. no. P2636)
Critical
We recommend using PLL for rat cultures. Alternatively, Poly-D-lysine hydrobromide (Sigma-Alrich, cat. no. P7280) can be used.
-
Boric acid (Sigma-Aldrich, cat. no. B6768)
Caution
Boric acid is hazardous. Wear appropriate eye and skin protection.
-
Sodium tetraborate (Sigma-Aldrich, cat. no. 221732)
Caution
Sodium tetraborate is toxic. Wear appropriate eye and skin protection.
-
UltraPure DNase/RNase-Free dH2O (Invitrogen-Thermo Fisher, cat. no. 10977015)
Neuronal dissection
-
Embryonic day 18 (E18) Sprague Dawley rats, specific-pathogen-free. In our experience, a pregnant Sprague Dawley dam carries a litter of 10–12 pups on average, which is sufficient to plate five to six 75-cm2 flasks for culturing a mix of cortical neurons and astroglial cells. This will typically yield 15 ml of neuroglial conditioned medium (NGCM) from a 75-cm2 flask. In order to obtain meaningful results, we recommend mixing of hippocampal neurons from at least five embryos per pregnant dam for one set of sdTIM experiments (six plates). We recommend performing at least three independent neuron dissections
Caution
All procedures using animals must be carried out in accordance with relevant institutional and governmental ethical guidelines and regulations. Permission to carry out the animal experiments described in this protocol was given by Animal Ethics Approval QBI/254/16/NHMRC.
-
Hank's buffered salt solution (HBSS) 10×, no calcium, no magnesium and no phenol red (Gibco-Thermo Fisher, cat. no. 14185-052)
-
HEPES, 1 M (Gibco-Thermo Fisher, cat. no. 15630-080)
-
Penicillin–streptomycin, 10,000 U ml−1 (Invitrogen-Thermo Fisher, cat. no. 15140-122)
-
Trypsin, 2.5% (wt/vol) (Gibco, cat. no. 15090-046)
-
Deoxyribonuclease I from bovine pancreas (DNase I; Sigma-Aldrich, cat. no. D5025-375KU)
-
FBS (Gibco, cat. no. 26140-079)
Caution
Wear appropriate eye and skin protection when using FBS.
Neuronal culturing
-
Neurobasal medium (Gibco-Thermo Fisher, cat. no. 21103-049)
-
B27 supplement, serum-free, 50× (Gibco-Thermo Fisher, cat. no. 17504-044)
-
GlutaMAX supplement, 100× (Gibco-Thermo Fisher, cat. no. 35050-061)
-
Cytosine β-D-arabinofuranoside (Ara-C; Sigma-Aldrich, cat. no. C1768)
-
Penicillin–streptomycin, 10,000 U ml−1 (Invitrogen-Thermo Fisher, cat. no. 15140-122)
-
HBSS (Gibco, cat. no. 14170-112)
DNA constructs
-
VAMP2-pHluorin (available upon request from J. Rothman, Yale University22). We use VAMP2-pHluorin to study SV recycling (see Experimental design section for more details)18. pHluorin22 can potentially be cloned to any plasma membrane protein destined for endocytic internalization to be used for sdTIM experiments (Fig. 2; Supplementary Table 1).
Primary neuron transfection
-
Lipofectamine 2000 (Invitrogen, cat. no. 11668027)
-
Neurobasal medium (Gibco-Thermo Fisher, cat. no. 21103-049)
Antibodies
-
Anti-synapsin-1 (Synaptic Systems, cat. no. 106011)
-
Anti-PSD-95 (Abcam, cat. no. ab99009)
-
Anti-mouse Alexa Fluor 546 (Abcam, cat. no. A11030)
EQUIPMENT
Microfluidic devices
-
Critical
The microfabrication of the device requires a clean room and soft lithography facilities.
Software for microfluidic mask design (Cadence (https://www.cadence.com/content/cadence-www/global/en_US/home/tools/custom-ic-analog-rf-design/circuit-design/virtuoso-schematic-editor.html), L-Edit (https://www.mentor.com/tannereda/l-edit) and Layout Editor (http://www.layouteditor.net/download.php5) GDS editors)
-
Reactive ion etch plasma system (Oxford Instruments, PlasmaPro 80 Reactive Ion Etcher model)
-
Spin coater (Brewer Science, model no. Brewer 200x)
-
Mask aligner (EV Group, model no. EVG620 Automated Mask Alignment System)
-
Mask writer (Heidelberg, model no. μPG101)
-
5-inch Chrome–soda glass photoplate (Clean Surface Technology, cat. no. CBC5009BU-AZ1500)
-
Hot plate(s) (e.g., Heidolph, MR Hei-End with 2 Lemo-Con model)
-
Fume hood (e.g., Science 2 Medical, standard PVC fume cupboard)
-
Sonicator (optional; e.g., Unisonics, model no. FXP20MH)
-
Vacuum desiccator (e.g., Cole-Parmer, model no. EW-06525-22)
-
100-mm Silicon wafers (University Wafer, cat. no. 590)
-
35-mm Glass-bottom cell culture dishes (Cellvis, cat. no. D35-20-1.5N or equivalent)
-
150-mm Petri dish (Corning, cat. no. 430597)
-
Clean glassware (crystallizing dish; e.g., Pyrex; Sigma-Aldrich, cat. no. CLS3140125)
-
Kimwipes (Kimtech Science, cat. no. 34155)
-
3M Scotch Magic Tape (3M, cat. no. Scotch Magic Tape 810)
3D printing of custom-made perfusion lid and preparation of perfusion chamber
-
35-mm Glass-bottom cell-culture dishes (Cellvis, cat. no. D35-20-1.5N or equivalent)
-
3D Builder (Microsoft, https://www.microsoft.com/en-us/store/p/3d-builder/9wzdncrfj3t6)
-
3D Printer control software (DataTree3D, https://datatree3d.com/software/)
-
2-mm Clear vinyl tubing (Pirtek, cat. no. CVT/10-2)
-
10-ml Syringes (e.g., Nipro, cat. no. 2183154)
-
Fume hood with UV light (UV sterilization cabinet, e.g., Wish-Med, model no. UVC-01)
Caution
Do not look straight into UV light.Wear protective eye and skin wear.
Assessment of fluorescent labeling of ligands and number of internalized ligands
-
Glass slides (Thermo Scientific, cat. no. 2012-04)
-
Coverslips (Thermo Scientific, cat. no. G401-10)
-
Coverslip tweezers (e.g., Dumont, cat. no. T04-821)
-
Double-sided tape (e.g., 3M Scotch 136 Double Sided Tape (12.7mm x 6.3 mm))
-
Compressed air (e.g., Corporate Express, cat. no EXP733)
-
Sonicator
Buffer preparation
-
Clean glassware (e.g., Pyrex)
-
Analytical scale (Mettler Toledo, model no. MS-TS)
-
Vapor-pressure osmometer (Wescor, model no. 5520)
-
pH meter (Mettler Toledo, model SevenCompact)
-
Magnetic stirrer (e.g., Heidolph, MR Hei-End with 2 Lemo-Con model)
-
0.22-μm Syringe-driven filter unit (Millipore, cat. no. SLGP033RS)
-
Syringes (Sigma-Aldrich, cat. no. Z118400-30EA)
-
Nalgene rapid-flow sterile disposable filter units with PES membrane (Thermo Scientific, cat. no. 167-0045)
Neuronal dissections and culturing
-
5-mm Biopsy punch (ProSciTech Rapid-Core biopsy punch, cat. no. T983-50)
-
Scalpel handle and blade (Generic; e.g., ProSciTech, cat. no. L055 and T133-2, respectively)
-
Laminar flow hood able to accommodate a dissecting microscope (e.g., Email Air, cat. no. 1687-0200/612-2)
-
Tissue culture incubator at 37 °C with a humidified, 5% CO2 atmosphere (e.g., Sanyo, model no. MCO-18AiC)
-
Water bath set to 37°C (Thermo Scientific, model no. Precision GP10)
-
Dissecting microscope (Olympus, model no. SZ51)
-
35-mm Glass-bottom cell culture dishes (Cellvis, cat. no. D35-20-1.5N or equivalent)
-
Nunc cell-culture-treated EasYFlasks, 75 cm2 (Gibco-Thermo Fisher, cat. no. 156499)
-
Dissecting tools (sterilized): dissecting scissors, dissecting forceps (e.g., Adson forceps, 1.2-mm tip, 125 mm), fine-tipped tweezers (e.g., Dumont tweezers, styles 5 and 5/45) and scalpel handle blade
-
Sterile plasticware: 5-, 10- and 25-ml serological pipettes; 60-mm tissue culture dishes and 15-ml and 50-ml conical centrifuge tubes; 2-, 20-, 200- and 1,000-μl filter tips; 1.5-ml Eppendorf tubes (e.g., Corning, Falcon, Maximum Recovery, Axygen)
-
P2, P20, P200 and P1000 micropipettes (e.g., Gilson)
-
Sterile glass Pasteur pipettes (Duran Wheaton Kimble, model no. 832)
-
Vacuum system for aspiration
-
Microcentrifuge (Beckman Coulter, model no. Microcentrifuge 18)
-
Hemocytometer for counting cells (e.g., Hausser Scientific, cat. no 3500)
-
Timer (Cole-Parmer, cat. no. EW-08649-10)
Super-resolution microscopy
-
Roper iLas2 microscope (Roper Scientific)
-
CFI Apo TIRF, 100×/1.49 NA oil-immersion objective (Nikon) with ×1.5 additional magnification
-
Electron-multiplying charge-coupled device (EMCCD) camera (Photometrics, model no. Evolve 512 Delta)
-
QUAD beam splitter (Semrock, cat. no. LF 405/488/561/635-A-00-ZHE)
-
QUAD band emitter (Semrock, cat. no. FF01-446/510/581/703-25)
-
MetaMorph software for image acquisition (MetaMorph Microscopy Automation and Image Analysis Software, v7.7.8; Molecular Devices, https://www.moleculardevices.com/systems/metamorph-research-imaging/metamorph-microscopy-automation-and-image-analysis-software)
-
Custom-made experimental perfusion and aspiration system for 35-mm glass-bottom cell culture dishes. The perfusion lid was designed to precisely fit to the 35-mm glass-bottom cell culture dish, which fits our microscope setup (Supplementary Fig. 1). To our knowledge, there are no commercially available perfusion lids with the designed specifications. We provide the 3D-print design in Supplementary Data 3
Critical
Similar perfusion systems are commercially available (e.g., PeCon, POC Cell Cultivation System).
-
10-ml Syringes
-
Water bath set to 37 °C for buffers
-
Timer
-
Immersol 518F (Zeiss, cat. no. 12-624-66A)
-
Inverted confocal microscope (Zeiss, model no. LSM 510 META) with Plan-APO 63×/1.4 NA oil-immersion objective, argon 488-nm and diode-pumped solid-state (DPSS) 561-nm lasers, and Zen 2009 software
-
Inverted confocal/two-photon microscope (Zeiss, model no. LSM 710) with 63×/1.4 NA oil-immersion objective, argon 488-nm and DPSS 561-nm lasers, gallium arsenide phosphide (GaAsP) detectors and Zen 2012 SP2 software
Analysis programs
-
Fiji85/ImageJ86 (National Institutes of Health, v2.0.0-rc-43/v1.50e, https://imagej.net/Downloads) for the analysis of nanobody fluorophore-labeling intensity, estimation of the number of internalized probes in an endocytic structure, color coding of super-resolved images, and brightness–contrast adjustment of images
-
PALMTracer49,50 (custom-written plug-in for MetaMorph) or TrackMate87 (https://imagej.net/TrackMate;standard plug-in for Fiji) for single-molecule tracking
Critical
Alternatively, use any freely available software for spt and develop analysis routines in order to obtain single-molecule-mobility parameters (see Experimental design).
REAGENT SETUP
1 M HEPES, pH 8.0
-
Dissolve 11.90 g of HEPES in 40 ml of Milli-Q water, place a pH electrode in the solution and, while mixing the solution with a magnetic stirrer, adjust the pH to 8.0 with NaOH. Bring the total volume to 50 ml with Milli-Q water. Store the stock solution at room temperature (i.e., 22–25 °C) for up to 6 months.
5 M NaCl
-
Dissolve 14.61 g of NaCl in Milli-Q water to a total of 50 ml. Store the stock solution at room temperature for up to 6 months.
Critical
5 M NaCl stock solution is very close to the saturation point of NaCl. As a consequence, the final volume must be carefully adjusted in order to allow the NaCl to be dissolved, which can take some time.
1 M KCl
-
Dissolve 11.9 g of KCl in Milli-Q water to a total of 50 ml. Store the stock solution at room temperature for up to 6 months.
1 M CaCl2
-
Dissolve 7.35 g of CaCl2 in Milli-Q water to a total of 50 ml. Store the stock solution at room temperature for up to 6 months.
1 M MgCl2
-
Dissolve 10.17 g of MgCl2 in Milli-Q water to a total of 50 ml. Store the stock solution at room temperature for up to 6 months.
560 mM D-glucose
-
Dissolve 5.04 g of D-glucose in Milli-Q water to a total of 50 ml. Store the stock solution at −20 °C for up to 6 months.
0.5 M ascorbic acid
-
Dissolve 4.40 g of ascorbic acid in Milli-Q water to a total of 50 ml. Store the stock solution at −20 °C for up to 6 months.
10% (wt/vol) BSA
-
Dissolve 5.0 g of BSA in Milli-Q water to a total of 50 ml. Store the stock solution at −20 °C for up to 6 months.
SU8-2005 dilution
-
Clean a fresh, sealable, glass100-ml container with acetone and isopropanol. Rinse the container with SU8-2000 thinner and remove the excess liquid. On a scale, add 40 g of SU8-2005 to the container. Add 10 g of SU8-2000 thinner to the container. Gently mix the solution to ensure a homogeneous mixture. Allow the mixture to rest for at least 12 h to ensure that no bubbles are present.
Caution
SU8-2005 thinner, SU8-2000 thinner, acetone and isopropanol are flammable and may cause skin and eye irritation. Avoid contact with the skin, eyes and clothing, and handle with gloves in a chemical fume hood.
Critical
All steps must be performed in clean-room facilities.
Critical
The SU8-2005 solution can be freshly prepared or stored at 4–22 °C, in a dark and dry environment until the expiration of the SU8-2005.
Preparation of PLL stock solution and dilutions
-
Prepare PLL stock solution by diluting 10 mg of PLL in 1 ml of 0.1 M borate buffer (pH 8.5; see below) to a final concentration of 10 mg ml−1. Filter-sterilize the solution with a syringe and a 0.22-μm filter. Divide the stock solution into 1-ml aliquots and store them at −20 °C for up to 2 months. For coating of plastic surfaces, such as a 75-cm2 flask, dilute the stock solution to a final concentration of 0.1 mg ml−1 by pipetting 0.1 ml of the 10-mg ml−1 stock solution into 9.9 ml of 0.1 M borate buffer (pH 8.5) and mixing the solution well. This dilution can be divided into aliquots and stored at −20 °C for up to 2 months. For glass-bottom dishes and microfluidic devices, prepare 1 mg ml−1 PLL stock solution by pipetting 1 ml of the 10-mg ml−1 stock solution into 9 ml of 0.1 M borate buffer (pH 8.5) and mixing the solution well.
Low-K+ and high-K+ buffers
-
To prepare low-K+ buffer (0.5 mM MgCl2, 2.2 mM CaCl2, 5.6 mM KCl, 145 mM NaCl, 5.6 mM D-glucose, 0.5 mM ascorbic acid, 0.1% (wt/vol) BSA and 15 mM HEPES, pH 7.4), mix 1.5 ml of 1 M HEPES at pH 8.0, 2.9 ml of 5 M NaCl, 560 μl of 1 M KCl, 220 μl of 1 M CaCl2, 50 μl of 1 M MgCl2, 1 ml of 560 mM D-glucose, 100 μl of 0.5 M ascorbic acid and 1 ml of 10% (wt/vol) BSA, adjust the pH to 7.4 with HCl and the total volume to 100 ml with Milli-Q water. Use a magnetic stirrer for efficient mixing of the solutions, especially during the pH adjustments. Prepare a high-K+ buffer similarly, with the exception of using 56 mM KCl (5.6 ml of 1 M stock solution) and 95 mM NaCl (1.9 ml of 5 M stock solution).
Critical
Measure the osmolarity using an osmometer; the osmolarity should be between 290 and 310 mOsm. Filter-sterilize and store the buffers at 4 °C for up to 2 weeks.
Preparation of Atto647N-NBs dilutions
-
For analyzing the ligand fluorophore labeling in cell-free systems, prepare 200 μl of 3.20 pg μl−1 dilution (1/100,000 dilution from 0.32 μg μl−1) of Atto647N-NBs in PBS at pH 7.4 (or pH 5.0 for acidic environment tests) in an Eppendorf tube, and use immediately. For activity-dependent internalization of VAMP2–pHluorin-bound Atto647N-NBs to study SV mobility in live hippocampal neurons, prepare a 3.20 pg μl−1 dilution of anti-GFP Atto647N-NBs in 10 ml of prewarmed (37 °C) high-K+ buffer in a 15-ml Falcon tube. Alternatively, you can use the same concentrations of anti-GFP Atto565-NBs or, if you are performing dual-color imaging, a combination of 3.20 pg μl−1 Atto565-NBs and 50 ng ml−1 Alexa647-CTB or another appropriate combination or ligand.
Critical
We recommend splitting the labeled nanobodies into 10- to 20-μl aliquots. Store the aliquots at −80 °C for up to 12 months and avoid repetitive freeze–thaw cycles. Freshly prepare the dilutions for each experiment.
Critical
Atto647N-NBs and Atto565-NBs are light-sensitive, and the stock solutions and the dilutions should be protected from light, e.g., by wrapping the tubes in foil.
Critical
For experiments in an acidic environment or at a pH other than neutral, titrate the PBS with HCl to pH 5.0 or to an appropriate pH and then use the solution immediately.
Critical
We recommend quantifying the fluorophore-labeling density of the ligands for each patch of the labeled, or third-party-provided, nanobodies or other ligands.
0.1 M borate buffer (pH 8.4)
-
Dissolve 3.09 g of boric acid, 4.77 g of sodium tetraborate and 2.19 g of NaCl in 450 ml of Milli-Q water. Mix the solution well with a magnetic stirrer while heating. Adjust the pH to 8.4 and filter-sterilize the solution with a sterile disposable filter unit. Store the buffer at 4 °C for up to 1 month.
Dissection medium
-
Prepare the dissection medium (DM), which is HBSS (1×) supplemented with penicillin (100 U ml−1)–streptomycin (100 μg ml−1) and HEPES (10 mM, pH 7.3), by mixing 100 ml of HBSS (10×) with 10 ml of (10,000 U ml−1) penicillin–(10,0000 μg ml−1) streptomycin and 10 ml of 1 M HEPES. Bring the volume to 1 liter with Milli-Q water. Filter-sterilize the solution with a sterile disposable filter unit and store the medium at 4 °C for up to 1 month.
Mix culture medium
-
Prepare 50 ml of neurobasal medium supplemented with penicillin (100 U ml−1)–streptomycin (100 μg ml−1), GlutaMAX supplement (1×), FBS (10%) and B27 (1×) by mixing 0.5 ml of penicillin (10,000 U ml−1)–streptomycin (10,0000 μg ml−1), 0.5 ml of GlutaMAX Supplement, 5 ml of FBS and 1 ml of B27; bring the volume to 50 ml with neurobasal medium. Mix the solution well and filter-sterilize if necessary. Mix culture medium (MCM) can be stored at 4 °C up to the manufacturer's expiration date.
Neuronal plating medium
-
Prepare 50 ml of neurobasal medium supplemented with penicillin (100 U ml−1)–streptomycin (100 μg ml−1), GlutaMAX supplement (1×) and FBS (5%) by mixing 0.5 ml of penicillin (10,000 U ml−1)–streptomycin (10,0000 μg ml−1) and 0.5 ml of GlutaMAX supplement, 5 ml of FBS; bring the volume to 50 ml with neurobasal medium. Mix the solution well and filter-sterilize if necessary. Neuronal plating medium (NPM) can be stored at 4 °C up to the manufacturer's expiration date.
Neuronal culture medium
-
Prepare 50 ml of neurobasal medium supplemented with penicillin (100 U ml−1)–streptomycin (100 μg ml−1), GlutaMAX supplement (1×), B27 (1×) and NGCM (20%) by mixing 0.5 ml of penicillin (10,000 U ml−1)–streptomycin (100,000 μg ml−1), 0.5 ml of GlutaMAX supplement, 1 ml of B27 and 10 ml of NGCM; bring the volume to 50 ml with neurobasal medium. Mix the solution well and filter-sterilize, if necessary. Neuronal culture medium (NCM) can be stored at 4 °C up to the manufacturer's expiration date.
Neuronal maintenance medium
-
Prepare 50 ml of neurobasal medium supplemented with penicillin (100 U ml−1)–streptomycin (100 μg ml−1), GlutaMAX supplement (1×) and B27 (1×) by mixing 0.5 ml of penicillin (10,000 U ml−1)–streptomycin (100,000 μg ml−1), 0.5 ml of GlutaMAX supplement and 1 ml of B27; bring the volume to 50 ml with neurobasal medium. Mix the solution well and filter-sterilize if necessary. Neuronal maintenance medium (NMM) can be stored at 4 °C up to the manufacturer's expiration date.
4% (wt/vol) Paraformaldehyde
-
Prepare 4% (wt/vol) PFA in PBS by pipetting 0.5 ml of 16% (wt/vol) PFA into 1.5 ml of PBS in a 2-ml Eppendorf tube. Store the 16% stock solution according to the manufacturer's instruction, and use freshly prepared fixative for best results.
Caution
PFA is a tissue fixative. Handle it with appropriate protective clothing, gloves and goggles. Work in a fume hood and discard by following institutional regulations.
EQUIPMENT SETUP
Single-molecule-microscopy setup for sdTIM experiments
-
We recommend using a 100×/1.49 NA oil-immersion objective with 1.5× additional magnification for single-molecule imaging. We recommend visualization at 50 Hz by acquiring between 10,000 and 20,000 frames by image streaming (we typically acquire 16,000 frames with a 20-ms exposure time) while maintaining the temperature constant at 37 °C. The dimensions of the images can be adjusted according to the region of interest (ROI; we typically use a 256 × 256-pixel image size).
Single-molecule tracking
-
Extract single-molecule localizations and trajectories from 16,000-frame oblique-illumination acquisitions, as described previously50, using a custom-made plug-in that runs within the MetaMorph program (or a complementary tracking method described previously88). Detect and track single endocytic molecules using a combination of multiframe object correspondence with simulated annealing48. To minimize nonspecific background detection, include only tracks longer than 8 frames in the analysis. Distinguish the mobile and immobile fractions as described previously89.
Procedure
Fabrication of microfluidic devices
Timing 5 h 30 min
Critical Step
Here, we describe step-by-step procedures for photomask fabrication using in-house facilities (Steps 1–21). Alternatively, when mask-generation facilities are unavailable locally, prefabricated chrome-glass or chrome-quartz laser-written photomasks or high-resolution transparency photomasks can be purchased through a third-party supplier.
Critical Step
The in-house fabrication of the microfluidic devices requires a clean room and a soft-lithography facility.
-
1
Place a 5-inch chrome–soda glass photoplate in an optical mask writer.
-
2
Load the first mask.gds file (Supplementary Data 2) into the system and generate a negative tone mask.
-
3
Write the mask by using the generation wizard to automatically align the plate, focus the objective and write the mask with 91% power.
-
4
Repeat Steps 1–3 for all three mask layers that are included in the .gsd file, sequentially (Supplementary Data 2).
-
5
Bake each photoplate for 1 min on a hot plate at 110 °C.
-
6
Allow each photoplate to return to room temperature for 10 min. This allows the thin resist layer to rehydrate with the atmospheric humidity.
-
7
In a fume cupboard, fill clean glassware with enough AZ 726MIF developer to completely submerge a photoplate.
Caution
The AZ 726MIF developer is TMAH-based, and it is corrosive and causes burns upon exposure to the skin. Use it only in a chemical fume hood with proper personal protective equipment.
-
8
Position the first photomask in the AZ 726MIF developer and allow it to develop completely, as observed by the naked eye, until the microfluidic structures are clearly visible. This will take ∼1 min.
-
9
Inspect the photoplate with an upright optical microscope with a 5× or 10× objective to ensure that the pattern is fully developed.
-
10
Rinse the photoplate with copious amounts of Milli-Q water and dry it with a nitrogen flow.
-
11
Repeat Steps 8–10 for each plate. The developer can be reused for each mask.
-
12
In a fume cupboard, add enough chromium etchant to fresh, clean glassware to fully submerge the photoplates.
Caution
Chromium etchant is a nitric acid, and ceric ammonium-nitrate-based etchant is corrosive and causes burns by all exposure routes. Use it only in a chemical fume hood with proper personal protective equipment.
-
13
Submerge the photoplate in the chromium etchant to etch the chromium layer to generate the photomask by optical inspection. Initially, the microfluidic features will appear to be a bright metallic color (after a few seconds of submersion); they will be completely transparent when complete. This step will take ∼1 min.
Critical Step
The last few Ångstroms of chromium take the longest to etch and can markedly affect the exposure dosage if they are not fully removed.
-
14
Rinse the photomask with copious amounts of Milli-Q water and dry it with a nitrogen flow.
-
15
Inspect the photomask with an upright optical microscope with a 5× or 10× objective to ensure that the chromium is fully etched in the patterned region. Incompletely etched chromium will appear dark and not completely transparent.
-
16
Repeat Steps 13–15 for each photomask, reusing the chromium etchant.
-
17
Add enough acetone to fresh, clean glassware to fully submerge the photomask.
Caution
Acetone is flammable and it may cause skin and eye irritation. Avoid contact with the skin, eyes and clothing, and handle it with gloves in a chemical fume hood.
-
18
Sonicate the photomask in acetone for 5 min.
-
19
Rinse the photomask, first with acetone and then with isopropanol.
Caution
Isopropanol is flammable and it may cause skin and eye irritation. Avoid contact with the skin, eyes and clothing, and handle it with gloves in a chemical fume hood.
-
20
Dry the photomask with a nitrogen flow and store it for future use in a clean-room environment.
-
21
Repeat Steps 18–20 for each photomask, reusing the acetone, if desired.
Wafer cleaning
Timing 1 h
-
22
Wafers can be cleaned through the use of either a piranha etch (option A) or a reactive ion etching plasma (option B).
Proper wafer cleaning is essential to ensuring good adhesion of the SU8-2100 to the wafer and to ensuring successful photolithographic patterning of the device structures. For best results, use option A. However, in facilities that do not support a piranha etch, option B can be used.
-
A
Piranha cleaning • TIMING 1 h
-
i
Take a fresh 100-mm silicon wafer from a cassette.
-
ii
Place a clean 150-mm glass beaker in an acid-compatible fume cupboard.
-
iii
Pour 150 ml of concentrated sulfuric acid into the glass beaker.
Caution
Concentrated sulfuric acid is a strong acid and an oxidizer; it causes burns by all exposure routes. Use it only in a chemical fume hood with proper personal protective equipment.
-
iv
Dropwise, add 50 ml of 30% (wt/vol) hydrogen peroxide to create a piranha etch while gently mixing.
Caution
The addition of hydrogen peroxide to the sulfuric acid causes a highly exothermic reaction; it will cause the mixture to boil. This piranha solution will be highly reactive with any organics, and its contact with organics may be explosive. Extreme care must be taken and full personal protection must be worn (face shield, acid apron and acid gloves).
-
v
Place the wafer carefully in the piranha solution with stainless steel or Teflon tweezers.
-
vi
Leave the wafer in the piranha etch for 20–40 min under supervision.
-
vii
Using metal or Teflon tweezers, remove the wafer from the piranha etch and rinse it with copious amounts of Milli-Q water.
-
viii
Dry the wafer with a nitrogen flow.
-
ix
Allow the piranha mixture to cool for several hours before disposing it of per local regulations.
Caution
The piranha will continue to generate gaseous oxygen for several hours. If the waste is stored for disposal, never place a closed lid on the storage vessel.
-
x
Place the freshly cleaned wafer on a hot plate at 180 °C for at least 1 h before use to ensure a dehydrated surface. Cool the wafer with dry nitrogen from a nitrogen-flow gun and immediately proceed to Step 23.
-
i
-
B
Reactive ion etch • TIMING 1 h
-
i
Take a fresh 100-mm silicon wafer from a cassette.
-
ii
Load the wafer into the reactive ion etch plasma cleaner.
-
iii
Purge the chamber and pump it down until it reaches a base pressure of <50 mTorr.
-
iv
Add pure oxygen gas at a flow rate that maintains a working pressure of 200 mTorr.
-
v
Ignite the plasma with a power of 200 mW for 5 min.
-
vi
Vent the chamber and remove the wafer, and immediately proceed to Step 23 for master fabrication.
-
i
-
A
Master fabrication
Timing 8 h
-
23
Patterning the first layer of the master (Steps 23–27). Place and center a freshly cleaned wafer on a spin coater, and fix its position by applying the vacuum. Add ∼5 ml of SU8-2100 photoresist on the center of the wafer.
Critical Step
Ensure that no bubbles are generated while pouring the SU8-2100 onto the wafer. Bubbles can create defects in the structures and affect subsequent layers.
-
24
Spin the wafer at 500 r.p.m. for 10 s, followed by 3,000 r.p.m. for 30 s at room temperature. Place the wafer on a hot plate at 65 °C for 5 min, and then move the wafer to a second hot plate at 95 °C for 1 h. Remove the wafer from the hot plate and allow it to cool to room temperature.
-
25
Load the first mask into the mask aligner, align the mask to be completely horizontal and expose it to a constant dose of 350 mJ cm−2 of UV (365 nm) light.
Caution
Do not look straight into UV light. Wear eye and skin protection.
-
26
Remove the wafer and immediately place it on a hot plate at 65 °C for 5 min.
-
27
Move the wafer to a hot plate at 95 °C for 30 min. Remove the wafer from heat and allow it to cool to room temperature.
Critical Step
The pattern of the first layer should be visible. This layer should define the first layer of the master and it should be ∼100 μm in height. If the height is inaccurate, the spin speeds in Step 24 may need to be adjusted. This layer defines the base of the device, as well as providing alignment marks for the subsequent layers.
-
28
Patterning the second layer of the master (Steps 28–31). Place and center the wafer that was patterned in Step 23 on the spin coater. Pour 5 ml of diluted SU8-2005 onto the wafer. Spin the wafer at 500 r.p.m. for 10 s and then at 4,000 r.p.m. for 30 s at room temperature.
Caution
SU8-2005 is flammable and it may cause severe skin and eye irritation. Avoid contact with the skin, eyes and clothing, and handle it with gloves in a chemical fume hood.
-
29
Remove the wafer and place it on the 65 °C hot plate for 1 min. Move the wafer to the 95 °C hot plate for 2 min. Remove the wafer from the heat and allow it to cool to room temperature.
-
30
Load the mask into the mask aligner. Align the first layer (Step 25) with this second layer. Align the mask horizontally. Load the wafer into the mask aligner. Align the wafer angle by focusing on the first layer of the wafer and leveling the alignment marks. Place the cross on the mask inside the box of the patterned SU8-2005 box on each side of the wafer. Expose the wafer to a constant dose of 100 mJ cm−2 of UV light.
-
31
Place the wafer on the 65 °C hot plate for 1 min. Move the wafer to the 95 °C hot plate for 2 min. Remove the wafer from the heat and allow it to cool to room temperature.
Critical Step
The pattern of the second layer should be visible. This should define the second layer of the master and the thickness should be 3 μm. Spin speeds in Step 28 should be adjusted if it deviates substantially from this size.
-
32
Patterning the third layer of the master (Steps 32–35). Place and center the wafer (that was previously patterned in Steps 23 and 28) on the spin coater. Pour 5 ml of SU8-2100 onto the wafer. Spin the wafer at 500 r.p.m. for 10 s and then at 3,000 r.p.m. for 30 s at room temperature.
-
33
Remove the wafer and place it on the 65 °C hot plate for 15 min. Move the wafer to the 95 °C hot plate for 1 h. Remove the wafer from the heat and allow it to cool to room temperature.
-
34
Load the photomask into the mask aligner. Align the mask horizontally. Load the wafer into the mask aligner. Align the wafer angle by focusing on the first layer of the wafer and leveling the alignment marks. The second layer alignment markers may or may not be visible. Place the small corner boxes on the mask inside the box of the patterned SU8-2100 box of the first layer on each side of the wafer. Expose the wafer to a constant dose of 400 mJ cm−2.
-
35
Place the wafer on the 65 °C hot plate for 5 min. Move the wafer to the 95 °C hot plate for 20 min. Remove the wafer from the heat and allow it to cool to room temperature.
Critical Step
The pattern of the third layer should now be visible. This should define the third layer of the master and the thickness should be 100 μm. Spin speeds in Step 32 should be adjusted if the third layer deviates substantially from this size.
-
36
Wafer development to create the master (Steps 36 and 37). Place two clean 150-mm beakers in a solvent-compatible fume cupboard. Pour ∼100 ml of PGEMA into one of the beakers. With metal tweezers, gently place the patterned wafer into the PGEMA. Agitate the PGEMA until all uncross-linked SU8-2100 is removed. This can be performed manually for 10–20 min or in a sonicator for ∼1 min. Rinse the master with fresh PGEMA. Rinse the wafer with copious amounts of isopropanol over the second beaker. Dry the master with nitrogen.
-
37
Visually inspect the master using an upright microscope with a 5× or 10× objective to ensure that all the unexposed SU8-2100 has been developed. If the wafer is not fully developed,there will be a white precipitate on the surface of the wafer after an isopropanol rinse. If the structure is not fully developed, it can be returned to the PGEMA (Step 36) for further development.
Silanization of the master
Timing 2 h
-
38
To silanize the master, place the master in an oxygen or air plasma cleaning unit. Pump down the chamber to ∼200 mTorr. Add oxygen or air to the chamber so that the working pressure stabilizes somewhere between 400 and 600 mTorr. Turn the power on high for 1 min. Vent the chamber.
-
39
Place the master in a vacuum desiccator. Place a Kimwipe in the vacuum desiccator and place a few drops of fluorosilane on the Kimwipe. Close the vacuum desiccator and leave the chamber connected to the vacuum for 5 min. Close the chamber and allow the master to remain in the pressure-reduced chamber for 30 min. Vent the chamber and remove the master. Bake the master for 1 h at 65 °C. Store the master in a clean environment until required.
Caution
Fluorosilane is corrosive and it causes burns by all exposure routes, especially through inhalation. It can react violently with water and produce highly toxic gases under fire conditions. Use it only in a chemical fume hood with proper personal protective equipment.
Critical Step
The master should be very hydrophobic at this point. This can be tested by placing a drop of Milli-Q water on the surface and ensuring that the drop rolls off the silicon surface.
Pause point
The master can be stored at room temperature for up to 1 year and should be reusable many times. The master should be discarded only when the features are mechanically damaged.
Soft lithography
Timing 1 h
-
40
Add 90 g of PDMS base mixture to a disposable plastic cup. Add 9 g of PDMS cross-linker and mix it vigorously for several minutes using a plastic spoon. Place the mixture in a vacuum desiccator under vacuum for 30 min.
-
41
Place the master fabricated in Step 39 in a 150-mm glass Petri dish. Pour the mixture over the master, and place the Petri dish in a vacuum desiccator for 30 min. Place a lid on the Petri dish to prevent ambient dust from settling into the PDMS.
-
42
Remove the Petri dish from the desiccator. Using a disposable Pasteur pipette, gently blow on the surface to pop any remaining bubbles. Place the Petri dish on a hot plate at 80 °C for 20 min. Remove the Petri dish from the hot plate and allow it to cool to room temperature. The PDMS should be fully hardened by now.
-
43
Cut the PDMS around the edge of the master using a scalpel and gently peel off the patterned device. Cut the PDMS piece into separate devices. Punch the four corner holes of each device with a 5-mm biopsy punch. Carefully cut around the edge of the device, ensuring that only the raised region of the ring remains. Clean each device with Scotch tape. Leave each device with the microfeatures covered with tape until it is time to bond the device.
Microfluidic device bonding
Timing 1 h
-
44
Remove the lid of a 35-mm glass-bottom Petri dish and place it in a plasma cleaner. Remove the Scotch tape from the PDMS device from Step 43 and place it in a plasma cleaner with the microfeatures facing up.
-
45
Pump the chamber down to a base pressure of at least 200 mTorr. Introduce oxygen or air so that the working pressure stabilizes between 400 and 600 mTorr. Ignite the plasma on high for 30 s. Vent the chamber.
-
46
Place the PDMS device onto the glass region of the glass-bottom cell-culture dish with the features facing down.
Critical Step
This step must be performed quickly without touching either surface, which will be bonded together.
-
47
Gently press the device against the dish. Place the lid back on top of the dish. Bake the devices overnight in an oven at 65 °C.
Sterilization and microfluidic device preparation
Timing 30 min
-
48
Place each of the devices from Step 47 into a large 150-mm glass Petri dish. Place this Petri dish into the plasma cleaner. Pump the chamber down to a base pressure of at least 200 mTorr. Introduce oxygen or air so that the working pressure stabilizes between 400 and 600 mTorr. Ignite the plasma on high for 2 min. Vent the chamber. Remove the devices and place them in a biosafety or laminar flow hood.
-
49
To prime the device, fill the well of the device with 70% (vol/vol) ethanol in one corner. Continue topping up the device with 70% (vol/vol) ethanol until it is filled hydrostatically on one side.
-
50
Let the device rest for 5 min to allow the ethanol to wick through the channels.
-
51
Repeat Step 49 with the other side of the device.
-
52
Rinse the device by aspirating the 70% (vol/vol) ethanol from the device ports and repeating Steps 49–51 with Milli-Q water.
-
53
Rinse the device by aspirating the Milli-Q water from the device ports and repeating Steps 49–51 with PBS. The device is now ready to be used or is coated with a biocompatible surface.
Pause point
We recommend using the microfluidic devices within a week after their preparation. If you are storing the microfluidic devices, seal the lid with Parafilm to prevent the PBS from evaporating and store them at 4 °C. In addition, you can add drops of UltraPure water to the periphery of the microfluidic devices to prevent the chambers from drying.
3D printing of custom-made perfusion lid and preparation of perfusion chamber
Timing 24 h
Critical Step
The perfusion lid (Supplementary Fig. 1) for the super-resolution-compatible glass-bottom cell culture dish was designed in-house using 3D Builder (Microsoft). The perfusion lid was designed to allow gas exchange (small adaptors in the lid) between the dish and the surroundings, as well as gentle liquid exchange in the cell culture dish. If the required facilities exist, the perfusion lid can be printed in-house after Steps 54–66. Alternatively, the 3D print can be obtained through an online 3D print service, such as i.materialise.com or 3dhubs.com.
-
54
Open the PerfusionLid.stl file (Supplementary Data 3) in Creation Workshop.
-
55
Position the model top down on the virtual build platform.
-
56
Slice the model into 50-μm slices by selecting a slice thickness of 0.05 mm under 'Configure Slicing Profile'.
-
57
Open the Kudo3D_Titan-2.0 program and initialize the printer. Turn on the fan and the projector.
-
58
Set z = zero for the build platform.
-
59
In the 'print' menu, open 'slices'. Set the z axis to 50 μm.
-
60
Set 'layer 1 exposure' to 40 s and 'lift' to 6 mm at 15 mm min−1.
-
61
Set the remaining layers to 12 s and the lift to 4 mm at 15 mm min−1.
-
62
Add the ABS-like resin to the tank and press print.
-
63
After printing, carefully separate the model from the build platform.
-
64
Rinse the model in absolute ethanol and then with tap water.
-
65
Dry the model and postcure it under UV light, under a laminar flow hood's sterilizer lamp or in direct sunlight, for 5 min on each side.
Caution
Do not look straight into UV light. Wear eye and skin protection.
-
66
Before use, insert a 2-mm tubing through the inlet and outlet holes. Adjust the depth of the hoses so that the outlet tube is placed close to the bottom of the cell-culture dish. The lid is now ready for use.
Pause point
Store the lid in a dust-free area until further use.
Flow-cell preparation and time-lapse imaging of the Atto647N-NBs
Timing 3 h
Critical Step
The following steps (Steps 67–72) describe how to obtain time-lapse acquisitions of the Atto647N-NBs, but they can be adapted without any special adjustments for the analysis of Atto565-NBs, Alexa647-CTB or other probes. We recommend carrying out Steps 67–78 for each of the labeled18 or third-party-provided NBs (or other fluorescently labeled ligands). The quantitation of the number of fluorophores per NB can then be used as an estimate for all subsequent tests, if the NBs are stored appropriately at −20 °C or −80 °C, covered from light and repetitive freeze–thaw cycles are avoided.
-
67
Place the glass slides (22 × 10 × 1 mm) and the rectangular, super-resolution-compatible coverslips in a glass beaker containing 1 M KOH and sonicate them for 20 min at room temperature to clean the glass surfaces.
Critical Step
Use plastic Pasteur pipettes in Steps 67and 68 to pipette the liquids up and down to ensure that the cover glasses do not stick together, therefore allowing efficient exchange of liquids.
-
68
Wash the glass slides and coverslips 5 times with an excess of Milli-Q water and dry them individually with air flow.
Critical Step
Steps 68–71 should be performed in a clean environment free of dust, such as a fume hood, and the cleaned coverslips should be handled only with tweezers.
Pause point
Dried glass slides and coverslips can be stored in a dry dust-free container for up to 1 month.
-
69
Once the glass slides are dry, assemble the flow chambers by attaching two strips of double-sided tape along the length of the slide. Align the cleaned rectangular coverslip with double-sided tape, and press the coverslip gently to create a proper seal to prevent leakage.
Critical Step
Take extra care when applying pressure to the coverslip edges to prevent them from breaking. Use a blunt object, such as the flat end of the tweezers, to attach the coverslip.
-
70
Prepare 200 μl of a 3.20 pg μl−1 dilution of Atto647N-NBs (or alternatively Atto565-NBs or Alexa647-CTB) in PBS at pH 7.4 (or pH 5.0, for acidic environment tests) (see Reagent Setup), pipette the dilution into the flow chamber and incubate it for 15 min in the dark to allow nanobody attachment to the glass surfaces.
Critical Step
Atto647N-NBs (or Atto565-NBs or Alexa647-CTB) are light-sensitive. Do not work under direct light, and cover the flow chamber during incubation to minimize bleaching.
-
71
Wash the flow chamber twice with PBS at pH 7.4 (or at pH 5.0 for acidic environment tests). For best results, aim for 50–100 nanobodies per 27 × 27 μm2. Transfer the flow chamber to the microscope.
-
72
Place a drop of immersion oil on an objective (100× 1.49 NA objective) in an inverted single-molecule-imaging microscope (see the Equipment and Experimental design sections for more detail) and place the flow chamber upside down on the glass slide holder. Adjust the objective position and optimize the focus, intensity and angle for oblique illumination of the excitation laser. Acquire time-lapse images from several regions with oblique illumination (50 Hz, 20-ms exposure, 16,000 frames). For short-term (i.e., a few minutes) high-frequency recording, use a 1.5× magnifier to expand the image on the camera. After acquisition, continue with Step 73.
Critical Step
To avoid bleaching of the fluorophores, it is critical to image areas that were not exposed to laser illumination. This can be achieved by finding the right focus and then switching off the laser. Then, move to the adjacent imaging area without turning on the laser. The laser should be turned on during the first frame or while taking the image stack.
Critical Step
To obtain comparable emission curves, it is important to use the same imaging setup for cell-free systems (Fig. 3a) as for live-cell experiments (Fig. 3b). Therefore, perform this step at 37 °C. To minimize bleaching, cover the samples to protect them from direct light and perform the recordings without a delay.
Obtaining emission curves and quantifying the fluorophore-labeling density of the Atto647N-NBs
Timing 1–2 h
Critical Step
The following Steps 73–78 describe the analysis of fluorophore labeling of anti-GFP Atto647N-NBs (Fig. 3a), but they can be adapted to the analysis of Atto565-NBs, Alexa647-CTB or other probes. Analysis can be done with ImageJ software or any equivalent image-analysis program. By quantifying the labeling density of the ligand, you can then proceed to estimate the number of ligands internalized in endocytic structures after sdTIM (described in Steps 115–125) in live and fixed neurons (Fig. 3b).
-
73
Open the data files obtained in Step 72 in ImageJ software.
-
74
Choose 5 × 5 pixels around the center of each Atto647N- or Atto565-NB fluorescent spot (Fig. 2a, red square) to obtain intensity–time traces using a custom-written routine as described previously90. Alternatively, the 'plot z axis profile' function in ImageJ/FIJI can be used. To obtain meaningful data, image and trace >100 fluorescent spots.
-
75
Subtract the background locally by quantifying the average intensity of a 2 × 2-pixel region surrounding each Atto647N- or Atto565-NB fluorescent molecule.
Critical Step
It is important that the signal-to-noise ratio be at least 1.5, so that the number of counted steps accurately reflects the number of fluorophores.
-
76
Exclude partially overlapping nanobodies and fluorophores that photobleached within the first two frames of the movies from the analysis.
-
77
Generate fluorescence intensity versus time traces for all remaining fluorescent spots. These traces have step-like features corresponding to bleaching of single molecules. Count the number of steps in the intensity–time traces manually. This distribution of steps reflects the number of fluorescent molecules per nanobody (Fig. 3).
-
78
Correct for missed events. In less than 2% of the traces, two molecules of Atto dyes will bleach within the same imaging frame. Such bleaching steps are twice the size of single-molecule steps and should be counted as two.
PLL coating for culturing a mix of cortical neurons and astroglial cells
Timing 2 h
Critical Step
Steps 79–81 must be performed in a cell-culture fume hood.
Critical Step
Never let PLL-treated surfaces dry.
-
79
Prepare a 10-mg ml−1 PLL stock dilution in 0.1 M borate buffer (pH 8.5) or thaw a previously prepared 1-ml aliquot that has been stored at −20 °C (see Reagent Setup). Dilute the 10-mg ml−1 PLL stock solution to a final concentration of 0.1 mg ml−1 in UltraPure water.
-
80
Coat a 75-cm2 plastic flask by pipetting 10 ml of the 0.1 mg ml−1 PLL dilution onto the bottom of the flask.
Critical Step
As neurons and glial cells adhere better to plastic than to glass surfaces, the concentration of PLL is lower (0.1 mg ml−1), and the duration of the coating is shorter (2 h) than when coating microfluidic devices or glass-bottom dishes, for which the PLL concentration is 1 mg ml−1 and the minimum incubation is 12 h (Steps 93–95).
-
81
Incubate the flask for 2 h at 37 °C, then remove the PLL solution and rinse the flask twice with UltraPure water.
Pause point
The coated 75-cm2 flasks can be stored in UltraPure water at 37 °C for up to 1 week. Make sure that the surface does not dry.
Preparation of NGCM from a mix of cortical neurons and astroglial cells
Timing 2–3 weeks
Critical Step
Steps 83–87 must be performed in a laminar-flow cabinet and Steps 88–92 must be performed in a cell-culture fume hood.
-
82
Chill the DM and prewarm (37 °C) all the cell culture media.
Critical Step
Keep the DM on ice throughout Steps 82–86, as it must remain ice-cold for optimal dissecting conditions. An experienced researcher can perform the dissection in Steps 83–86 quickly enough to prevent the DM from becoming warm. However, if required, the DM on the dish can be replaced with fresh ice-cold DM or the dish can temporarily be placed on ice to prevent temperature rise.
-
83
Sacrifice a pregnant (E17–18) dam using an approved method of euthanasia (e.g., cervical dislocation), dissect out the uterus and place it in a sterile 60-mm tissue-culture dish with ice-cold DM. In our experience, a pregnant Sprague Dawley dam carries a litter of 10–12 embryos on average, which is a sufficient number to plate five to six 75-cm2 flasks for culturing a mix of cortical neuron and astroglial cells. This will typically yield 15 ml of NGCM from each 75 cm2 flask.
Caution
All procedures using animals must be carried out in accordance with relevant institutional and governmental ethical guidelines and regulations. Permission to carry out the animal experiments described in this protocol was based on Animal Ethics Approval QBI/254/16/NHMRC.
-
84
Remove the E17-18 fetuses from the uterus. To obtain an optimal cell confluence, we recommend using two embryos for each 75-cm2 flask. Using a dissecting microscope placed in a laminar-flow cabinet, dissect out the brains and place them in a new dish containing ice-cold DM. The tissue must remain submerged at all times.
-
85
Using a scalpel, carefully dissect both forebrains from each brain and remove the meninges under a dissecting microscope. Collect the forebrains in a new dish containing ice-cold DM.
-
86
Manually triturate the forebrains using the scalpel. Collect the tissue and place it in a 15-ml conical centrifuge tube. Bring the volume to 4.7 ml with DM. Add 0.3 ml of 2.5% (wt/vol) trypsin and incubate the tube for 15 min in a water bath at 37 °C with regular agitation (every 2–3 min).
-
87
Stop trypsinization by adding 1.5 ml of FBS. Add 0.5 ml of 1% (wt/vol) DNase I and incubate the tube for 10 min in a water bath at 37 °C.
-
88
Homogenize by pipetting up and down using a P1000 pipette until no chunks are observed (typically 40–50 times).
-
89
Centrifuge the cells for 8 min at 120g at room temperature. Discard the supernatant and suspend the pellet in 1 ml of prewarmed (37 °C) MCM. Pipette up and down 10 times with a P1000 pipette. Add another 9 ml of prewarmed (37 °C) MCM.
-
90
Plate the cells from one forebrain into a PLL-coated 75-cm2 flask from Step 81 and add prewarmed (37 °C) MCM to a final volume of 15 ml per flask. Place the neurons in a tissue culture incubator at 37 °C with 5% CO2.
-
91
Change the medium every 2–3 d. Before removing the old medium, swirl it to remove loosely attached cells.
-
92
After 7 d, remove the MCM and add 10–15 ml of prewarmed (37 °C) NMM. To completely remove the FBS, the culture can be washed once with neurobasal medium before the addition of NMM. NGCM is ready to be harvested 10–14 d later. In the meantime, proceed to prepare PLL-coated microfluidic devices and glass-bottom dishes (Step 93 and further).
Pause point
NGCM can be added straight to the appropriate neuronal culture medium or can be collected, divided into aliquots and stored at −20 °C to −80 °C for up to 6 months.
PLL coating of microfluidic devices and glass-bottom dishes
Timing 24 h
Critical Step
Steps 93–95 must be performed in a cell-culture fume hood.
Critical Step
Never let PLL-treated surfaces dry.
-
93
Prepare a 10-mg ml−1 PLL stock solution in 0.1 M borate buffer (pH 8.5), or thaw a previously prepared 1-ml aliquot (see Reagent Setup), and dilute the stock dilution to a final concentration of 1 mg ml−1 in 0.1 M borate buffer (pH 8.5).
-
94
1–2 d before the dissection of hippocampal neurons (Steps 96–105), coat the microfluidic devices (or glass-bottom dishes) with 1 mg ml−1 PLL in 0.1 M borate buffer (pH 8.5) by pipetting 200 μl into both the soma and terminal wells of a microfluidic device (or by adding 350 μl to the glass-bottom part of the cell-culture dish). For efficient coating, incubate the microfluidic devices for minimum of 12 h at 37 °C. The incubation time for glass-bottom dishes can be shortened to a minimum of 3–4 h, if needed.
-
95
Remove the PLL solution and wash the flask 3 times with UltraPure water. Note that the 1 mg ml−1 PLL dilution from Step 94 can be collected, stored at −20 °C and reused once for coating of 75-cm2 flasks.
Critical Step
For efficient cleaning of the microchannels, incubate the microfluidic devices in water for at least 12 h. The incubation time for glass-bottom dishes can be shortened to a minimum of 3–4 h, if needed.
Pause point
The coated microfluidic devices (or glass-bottom dishes) can be stored at 37 °C (or 4 °C) for up to 3 d. If storing for more than 3 d, add drops of UltraPure water to the periphery of the microfluidic devices to prevent the chambers from drying.
Dissection and plating of hippocampal neurons to microfluidic devices or glass-bottom dishes
Timing 2–3 h
Critical Step
Steps 97–101 must be performed in a laminar flow cabinet and Steps 102–105 in a cell-culture fume hood.
-
96
Chill the DM and prewarm (37 °C) all the media.
Critical Step
Keep the DM on ice throughout Steps 96–105, as it must remain ice-cold for optimal dissecting conditions. An experienced researcher can perform the dissection steps (Steps 97–100) quickly enough to prevent the DM from becoming warm. However, if needed, the DM on the dish can be replaced with fresh ice-cold DM, or the dish can be temporarily placed on ice to prevent temperature rise.
-
97
Repeat Steps 83–85 from the 'Preparation of NGCM from a mix of cortical neurons and astroglial cells' section and continue to Step 98.
Caution
All procedures using animals must be carried out in accordance with relevant institutional and governmental ethical guidelines and regulations. Permission to carry out the animal experiments described in this protocol was based on Animal Ethics Approval QBI/254/16/NHMRC.
Critical Step
The number of glial cells increases when the neurons are obtained from postnatal pups. We recommend using E17–E18 rat or mice embryos. To obtain meaningful results, we recommend mixing of hippocampal neurons from at least five embryos per pregnant dam for one set of sdTIM experiments (six plates) and performing at least three independent neuron dissections.
-
98
Dissect out the hippocampi using sterile fine-tipped tweezers.
-
99
Collect the hippocampi in a new dish containing ice-cold DM to ensure that the neurons are kept cold.
-
100
Collect all the hippocampi in an Eppendorf tube and bring the volume to 270 μl with ice-cold DM. Add 30 μl of 2.5% (wt/vol) trypsin and incubate the tube for 10 min in a 37 °C water bath with regular agitation (every 2–3 min).
-
101
Stop the trypsinization by adding 150 μl of FBS. Add 50 μl of 1% (wt/vol) DNase I and incubate the tube for 10 min in a 37 °C water bath.
-
102
Triturate by pipetting up and down using a P200 pipette until no chunks are observed (typically 40–50 times).
-
103
Centrifuge the cells for 8 min at 120g at room temperature. Discard the supernatant and resuspend the pellet in 100 μl of prewarmed (37 °C) NPM. Pipette up and down 10 times using a P200 pipette.
Critical Step
With experience, the researcher will be able to estimate the average number of neurons isolated per animal (per two hippocampi). This value will be very useful in choosing the appropriate resuspension volume.
-
104
Count the neurons using a hemocytometer and a cell counter. Check the viability using trypan blue exclusion. From each dissected hippocampus, up to 400,000 neurons can be obtained. Viability of the neurons depends on the experience of the researcher and how quickly the neuronal dissections are done. In our experience, we usually get 80–90% viability in our dissections.
-
105
Plate the neurons either in microfluidic devices (option A) or glass-bottom dishes (option B). Choose option A for polarized culturing of hippocampal neurons to assist with the discrimination of morphological features and identification of nerve terminals and axons. We recommend choosing option A particularly for studying long-range retrograde (or alternatively anterograde, i.e., toward the nerve terminal) transport in axons, such as for tracking Alexa647-CTB (and, when performing dual-imaging, Atto565-NBs). We recommend choosing option B with the custom-made perfusion system to perform the pulse–chase steps of the sdTIM method using Atto565-NBs or Atto647N-NBs. Note that although option B is most useful in studying SV mobility in presynapses and adjacent axons, it can also be used to track long-range trafficking in axons by tracing and recording the orientation of the neuron on the glass-bottom dish. Option B is also the best option for recording the VAMP2-pHluorin fluorescence.
-
A
Plating neurons in microfluidic devices
-
i
Aspirate the UltraPure water from the microfluidic devices and rinse them with NPM.
Critical Step
It is important to remove all the water from the microfluidic channels before plating the neurons by carefully washing the chambers with NPM. If uncertain that all the water has been removed, repeat the NPM wash 1–2 times.
-
ii
Make an appropriate dilution of neurons in prewarmed (37 °C) NPM in an Eppendorf tube.
Critical Step
The plating volume of the soma well (Fig. 1f (inset), red chamber) is ≤10 μl. The density of cells must be ≥5,000 neurons μl−1. If the density is lower, the neurons should be centrifuged again (Step 103) and suspended in an appropriate volume.
-
iii
Aspirate the NPM and plate 50,000 neurons in the soma well and 2,500–5,000 neurons in the terminal well by pipetting up and down slowly from both sides of the soma well. Pipetting is recommended only in the soma well to distribute the neurons close to the channels. In the nerve terminal well (Fig. 1f (inset), blue chamber), neurons should be sufficiently far from the channels to ensure that a minimum number of axons will reach the channels.
Critical Step
It is important to avoid creating air bubbles while pipetting, as they prevent the equal distribution of the neurons in the microfluidic devices and may block the neurons from being seeded close to the channel entrances.
-
iv
Place the neurons in a tissue-culture incubator at 37 °C with 5% CO2. After 20 min, check under a dissecting microscope whether the neurons have attached to the microfluidic devices and add 200 μl of prewarmed (37 °C) NPM. Continue with Step 106 after 2–4 h.
-
v
(Optional) Add small drops of UltraPure water to the edges of the microfluidic device dish to create a humid atmosphere and to prevent the chamber from drying out.
Critical Step
The humidity of the tissue culture incubator must remain constant at all times, as lowering the humidity facilitates drying of the microfluidic devices.
-
i
-
B
Plating neurons on glass-bottom dishes
-
i
Aspirate the UltraPure water and rinse with NPM.
-
ii
Make an appropriate dilution of neurons in NPM in an Eppendorf tube or a Falcon tube. We plate 50,000–100,000 neurons per dish. The plating volume for the glass bottom of the culture dishes is 0.5 ml.
-
iii
Aspirate the NPM and plate the neurons on the glass-bottom dishes.
-
iv
Place the neurons in a tissue-culture incubator at 37 °C with 5% CO2 and continue with Step 106 after 2–4 h.
Critical Step
The humidity of the tissue-culture incubator must remain constant at all times, as lowering the humidity facilitates drying of the glass-bottom dishes.
-
i
-
A
Culture and maintenance of hippocampal neurons
Timing 2–3 weeks
Critical Step
Steps 107–108 must be performed in a cell culture fume hood.
-
106
Prewarm NCM to 37 °C.
-
107
Carefully add 1.5 ml of NCM to the glass-bottom dishes. When using microfluidic devices, carefully remove all remaining NPM (∼150 μl) from both chambers and replace it immediately with 150 μl of NCM per chamber.
-
108
Every 7 d (or 3 d, when using microfluidic devices), replace 1/3 to 1/2 of the medium with fresh NMM.
Critical Step
Check whether the added drops of UltraPure water in the periphery of the microfluidic devices have evaporated, and add more water if needed. Be careful not to contaminate the microfluidic wells with water by using small drops of water (∼50 μl).
Critical Step
When using E18 rat embryos for neuronal dissections, the culture dishes should contain no more than 10–20% glial cells (as a percentage of the total cell count).
Primary neuron transfection in microfluidic devices and in glass-bottom cell-culture dishes
Timing 24–48 h
Critical Step
For the best transfection efficiency, we recommend transfecting E18 hippocampal neurons between days in vitro (DIV)14 and 16.
Critical Step
Perform Steps 109–114 in a fume hood.
-
109
Pipette 10 μl (or 100 μl) of room-temperature neurobasal medium into two 1.5-ml Eppendorf tubes, labeled 'A' and 'B'.
Critical Step
It is important to use Neurobasal medium without any supplements, particularly antibiotics, to create the transfection mixture. The transfection efficiency is very low if penicillin–streptomycin is used.
-
110
Add 1 μl (or 2 μl) of Lipofectamine 2000 transfection reagent to tube A and 1 μg (or 2 μg) of VAMP2-pHluorin, or another plasmid DNA, to tube B, and mix well by pipetting up and down. Incubate the tubes for 5 min at room temperature.
-
111
Combine the contents of tubes A and B, mix well and incubate for 30 min at room temperature.
-
112
Carefully collect the medium from the soma wells of the microfluidic devices in an Eppendorf tube (or from the cell-culture dishes in a 15-ml Falcon tube by leaving a residual volume of 380 μl in the glass-bottom part of the dish to cover the neurons) and store it at 37 °C.
Critical Step
It is very important to never remove all the medium from the neurons, as this could decrease the viability of the cells. A small volume of medium will remain in the neuronal chambers of the microfluidic devices when removing the medium from the wells, which protects the neurons from drying out; in the glass-bottom dishes, collect the medium from the plastic periphery surface, leaving some residual medium (∼380 μl) in the glass-bottom part. After removing the medium, immediately add the transfection mixture to the cells to avoid drying.
Critical Step
To help with the orientation of the microfluidic chamber under the microscope at later stages (Step 117B(iv)), make a small mark on the side of the soma wells with a permanent marker.
-
113
Add the transfection mixture to the neurons by carefully pipetting up and down from both sides of the soma well (or simply add the transfection mixture dropwise to the glass-bottom part of the dish) to allow thorough spreading of the reagent mixture. Incubate the dish for 2–5 h at 37 °C.
Critical Step
Try to avoid introducing bubbles into the microfluidic wells by pipetting up and down slowly.
-
114
Wash the soma well (or glass-bottom dishes) 3–5 times with fresh neurobasal medium by pipetting 150 μl (or 500 μl) into one of the soma wells (or into the glass-bottom dishes) and aspirating from the other and then replace the medium with the medium collected from the soma wells (or glass-bottom dishes) before transfection in Step 112. Incubate the dishes for 24–48 h and continue with Step 115.
Critical Step
It is very important to remove all Lipofectamine transfection reagent to avoid cell death.
Induction of synaptic activity and uptake of fluorescently labeled ligands
Timing 30 min—3 h per dish
Critical Step
The following steps describe the use of anti-GFP Atto647N-NBs in hippocampal neurons grown on glass-bottom dishes and transfected with VAMP2-pHluorin. If performing dual-color imaging, use a combination of anti-GFP Atto565-NBs and Alexa647-CTB, or another appropriate combination. Note that while Atto565-NBs can also be used for imaging SV mobility in presynapses, we recommend the use of Atto647N-NBs to obtain the best results if only imaging one compound.
Critical Step
To assess the correct localization of the internalized ligands (Box 1), anti-GFP HRP-mCherry-GFP nanotrap (GNT) nanobodies18 (10 μg ml−1) can be used as a ligand instead of fluorescent nanobodies (Step 117), followed by fixation, cytochemical staining and processing for EM as described earlier18,91,92.
-
115
Preheat the low-K+ and high-K+ buffers to 37 °C.
-
116
Prepare a 3.20 pg μl−1 dilution of anti-GFP Atto647N-NBs in 10 ml of prewarmed (37 °C) high-K+ buffer in a 15-ml Falcon tube (see Reagent Setup). Alternatively, you can use the same concentration of anti-GFP Atto565-NBs or, if performing dual-color imaging, a combination of 3.20 pg μl−1 Atto565-NBs and 50 ng ml−1 Alexa647-CTB. In addition, prepare a 10-ml aliquot of prewarmed (37 °C) high-K+ buffer in another 15-ml Falcon tube.
-
117
Induction of synaptic activity and uptake of fluorescently labeled ligands can be done either in glass-bottom dishes equipped with a custom-made perfusion lid (option A) or in microfluidic devices (option B). Use mature (Box 1) DIV15–17 hippocampal neurons expressing VAMP2–pHluorin when studying SV mobility in presynapses or axons.
-
A
Activity-dependent internalization of VAMP2–pHluorin-bound Atto647N-NBs for studying SV mobility in presynapses and axons
-
i
Remove the culture medium from the cell-culture dish from Step 114 by aspirating the volume from the plastic part of the dish.
Critical Step
All the steps involving liquid exchange in the glass-bottom dishes are performed by aspirating/injecting the liquids from/to the plastic part of the cell culture dish, leaving the glass bottom of the dish submerged (∼380 μl). This remnant volume will protect the neurons from drying and allows much more gentle exchange of liquid than pipetting straight onto neurons grown on the glass bottom. It is critical that all the liquid exchange is therefore done with an excess volume of liquids, taking into account the remaining volume in the glass-bottom part.
-
ii
Wash the cells 4–5 times with 2 ml of prewarmed (37 °C) low-K+ buffer. Transfer the chamber immediately to the microscope.
Critical Step
Transferring the culture dishes from the hood to the microscope should be done as quickly as possible to avoid excessive temperature changes on the plates.
-
iii
Place the glass-bottom cell culture dish in the microscope dish holder and replace the culture dish lid with the custom-made perfusion lid from Step 66 (Supplementary Fig. 1).
Critical Step
Make sure that the tubing passing through the perfusion lid does not touch the cell-culture dish bottom to avoid unnecessary vibration and disturbance. Make sure that the outlet tube for aspiration is placed close to the bottom of the dish to allow efficient liquid exchange.
-
iv
Locate a representative VAMP2-pHluorin-positive hippocampal neuron. Note that the retrograde and anterograde studies can also be carried out on a glass-bottom dish by tracing the orientation of the neuron, i.e., recording the location of the soma and axon. However, if possible, we would recommend using microfluidic devices.
-
v
Attach an empty 10-ml syringe to the end of the perfusion lid tubing and aspirate the low-K+ buffer from the dish carefully (remove ∼1,600 μl). Note that only the liquid above the glass bottom will be aspirated, leaving the glass bottom submerged in low-K+ buffer.
-
vi
Attach another 10-ml syringe containing 2 ml of prewarmed (37 °C) high-K+ buffer containing 3.20 pg μl−1 of Atto647N-NBs, and add this liquid to the neurons while recording the changes in the VAMP2-pHluorin fluorescence (acquire images at 50 Hz, 20-ms exposure time, 3,000–5,000 frames). Recording of VAMP2-pHluorin fluorescence will allow the identification of presynaptic active zones.
Critical Step
You can alternatively use high-K+ buffer containing Atto565-NBs or, if performing dual-color imaging, a combination of Atto565-NBs and Alexa647-CTB. To study SV mobility in presynapses, the concentration of the added ligand should be tightly controlled. When using an adaptation of sdTIM or different ligands, the concentrations can be adjusted based on your needs.
Critical Step
Liquid exchange in the cell-culture dish while imaging can cause changes in the focus, or even cause the neurons to move. The perfusion system described here is designed to minimize disturbances by injecting liquids onto the plastic portion of the dish only, which facilitates a more gentle exchange of the liquids on the glass-bottom portion of the dish, where imaging takes place.
-
vii
Replace the syringe containing the stimulation buffer with a 10-ml syringe containing 10 ml of prewarmed (37 °C) low-K+ buffer and attach an empty syringe to the aspiration tube. 5 min after applying the high-K+ buffer containing Atto647N-NBs (pulse), aspirate the high-K+ buffer from the plate (remove ∼1.6 ml) and inject 2 ml of prewarmed (37 °C) low-K+ into the plate. Repeat this five times to remove any traces of unbound Atto647N-NBs and incubate the plate for 10 min (chase) in total.
Critical Step
The duration of the stimulation should be tightly controlled. Note that if you are using an adaptation of sdTIM, you can change the pulse time according to your needs.
-
viii
After the 10-min chase, image the Atto647N-NB mobility with oblique illumination microscopy (similarly to Step 72) in the same region where you recorded the changes in VAMP2-pHluorin fluorescence (Step 117A(vi)). Note that if you are using an adaptation of sdTIM, you can change the chase time according to your needs.
Critical Step
After use, clean the perfusion lid by injecting UltraPure water through the tubing and then dry it by pressing air through the tubes to remove any remaining water. Replace the tubing if necessary.
Critical Step
Step 117A(i and ii) is carried out in a cell culture fume hood and Step 117A(iii–viii) is carried out at 37 °C under a microscope.
-
i
-
B
Concomitant internalization of VAMP2-pHluorin-bound Atto565-NBs and Alexa647-CTB for dual-color imaging
-
i
Collect the culture medium from the soma and terminal wells (∼150 μl each into a 1.5-ml Eppendorf tube) of the microfluidic devices from Step 114 with a P200 pipette and store the tube at 37 °C.
Critical Step
All the steps involving liquid exchange in the microfluidic devices are performed by aspirating the entire liquid volume from the soma and terminal wells, which will leave a remnant volume of liquid to cover the neurons in the soma and terminal chambers as well as the microfluidic devices. If promptly replenished with another liquid, the neurons will be protected from drying.
-
ii
Wash the soma and terminal wells once with 150 μl of prewarmed (37 °C) low-K+ buffer by using a P200 pipette. Transfer the microfluidic device immediately to the microscope.
-
iii
Place the microfluidic device in the microscope dish holder by orientating the soma wells on the right based on the mark made in Step 112.
Critical Step
Transferring the culture dishes from the hood to the microscope and back should be done as quickly as possible to avoid excessive temperature changes on the plates.
-
iv
Locate the microfluidic channels and find the hippocampal neurons passing through the channels. Neurons can be located either by using bright-field imaging or based on the VAMP2-pHluorin fluorescence.
Critical Step
Check the orientation of the selected neuron by tracing the cell to the soma and nerve terminals. Take note of the neuronal orientation in order to discriminate retrograde and anterograde transport. In addition, note the row number of the channel through which the neuron passes, so that it is easier to locate the same neuron after removing the dish from the microscope. This can be done by simply counting the rows from the top or the bottom of the chamber.
-
v
Carefully remove the low-K+ buffer from both the soma and the terminal wells with a P200 pipette without disturbing the device.
-
vi
Add 200 μl of prewarmed (37 °C) high-K+ buffer to the soma well and 100 μl of prewarmed (37 °C) high-K+ buffer containing 3.20 pg μl−1 Atto565-NBs and 50 ng ml−1 Alexa647-CTB to the terminal wells. Continue immediately with Step 117B(vii).
Critical Step
The concentration of the added Atto565-NBs and Alexa647-CTB should be tightly controlled. Note that the concentration of your ligand can be adjusted according to your needs, if you are using an adaptation of sdTIM.
Critical Step
Add Atto565-NBs and Alexa647-CTB to only the synaptic region, i.e., terminal wells, and not to the soma wells. To avoid any flux of buffer from the terminal well; the volume added in the soma wells must be higher (200 μl) than that in the terminal wells (100 μl). Consequently, any endocytic compartments recorded within the axons passing the channels must move in the retrograde direction (toward the soma).
-
vii
Stimulate the neurons for 5 min (pulse) in total at 37 °C under the microscope. Record the change in VAMP2-pHluorin fluorescence during the addition of high-K+ stimulation (if possible) by taking a short video or immediately after. If not recording the change of VAMP2-pHluorin fluorescence during the addition of high-K+ buffer to the chamber, we recommend taking a still image before stimulation, and comparing the mean VAMP2-pHluorin fluorescence before and after the pulse. This will provide enough information to identify active zones.
Critical Step
Changes in the VAMP2-pHluorin unquenching occur quickly after the stimulation, and imaging should therefore be done immediately.
Critical Step
Do not exceed 5 min of stimulation, unless you are using an adaptation of sdTIM. After recording the VAMP2-pHluorin unquenching, transfer the chamber back to the cell-culture fume hood within the 5-min stimulation.
-
viii
Remove any unbound Atto565-NBs and Alexa647-CTB and the remaining high-K+ buffer by washing the terminal and soma wells immediately three times with prewarmed (37 °C) low-K+ buffer (200 μl) in the cell-culture fume hood.
-
ix
Replace the low-K+ buffer with the collected culture medium (from Step 17B(i)).
-
x
Chase the neurons for 2 h at 37 °C in a cell incubator with 5% CO2. Note that the chase time can be adjusted based on your needs.
Critical Step
In the experiments to detect single internalized nanobodies in the SVs in nerve terminals at earlier time points, chase neurons for 10 min in total in low-K+ buffer to maximize the internalization of VAMP2-pHluorin-bound Atto565-NBs and then continue straight with the imaging using oblique illumination microscopy.
-
xi
After the 2-h chase, remove the culture medium from both wells by washing three times with prewarmed (37 °C) low-K+ buffer (200 μl).
-
xii
Transfer the microfluidic chamber back to the microscope and locate your neuron of interest (this can be done based on the notes from Step 17B(iv)). To detect single molecules of internalized Atto565-NB and Alexa647-CTB in the nerve terminals and/or axons passing through the channels, image the neurons using oblique illumination microscopy.
Critical Step
Step 117B(i–ii, viii, ix and xi) is carried out in a cell culture fume hood, Step 117B(x) in the 37 °C cell culture incubator in a 5% CO2 atmosphere and Step 117B(iv–vii and xii) at 37 °C under a microscope.
-
i
-
A
Single-molecule tracking with TrackMate
Timing 2 h
Critical Step
The following steps (Steps 118–124) describe single-particle-tracking analysis of internalized Atto647N-NBs using the ImageJ plug-in TrackMate87 but can be adapted to the analysis of Atto565-NBs, Alexa647-CTB or other ligands.
-
118
Open the time-lapse acquisitions of internalized nanobodies (Step 117A(viii) or 117B(xii)) in ImageJ software. If desired, a ROI can be drawn at this stage if only a subsection of the image should be analyzed.
Critical Step
In addition, we also recommend analyzing the time-lapse acquisition of fixed neurons (Step 125A).
-
119
Run the TrackMate plug-in.
-
120
Select the 'LoG detector', 'median filter' and 'subpixel localization' in the particle-detection process.
-
121
To link particles into traces, adjust the linking distance in the Simple LAP tracker while keeping the gap closing at zero. Note that the linking distance depends on time resolution and mobility of fluorescent spots. It is best to choose an exposure time, which prevents the Atto647N-NBs from moving distances greater than the diameter of the point spread function of the microscope between adjacent frames. Run the tracker.
-
122
TrackMate has several spot-filtering options listed in the filter menu. Filter particle traces based on trace length and discard traces containing fewer than eight spots.
-
123
Run 'Analysis' to generate the 'Spots in Tracks Statistics' text file. This file contains the x and y coordinates, as well as spot-intensity values, of the spots linked into each track, which are necessary to compute mean square displacement (MSD) curves and diffusion coefficients. The x and y coordinates are required for computing MSD plots and diffusion coefficients. The spot-intensity values are used in Step 125.
-
124
Run the provided custom-written MATLAB routine (Supplementary Data 1) to compute MSD curves and diffusion coefficients. Detailed instructions on how to use the MATLAB scripts are provided with the code. Presynaptic and axonal regions of interest can be manually selected as ROIs before tracking. This allows the discrimination of single-particle tracks of SVs originating from these distinct regions. Active zones can be discriminated from axons by following the acquired VAMP2-pHluorin fluorescence videos obtained in Step 117A(vi).
Estimating the number of internalized ligands in endocytic structures
Timing 4 h
-
125
Estimate the number of internalized ligands in fixed (option A) or live (option B) hippocampal neurons. Estimating the fluorophore-labeling density of the nanobodies is carried out following Steps 73–78 ('Obtaining emission curves and quantifying the fluorophore-labeling density of the Atto647N-NBs'). Note that the following steps describe the analysis of VAMP2-pHluorin-bound anti-GFP Atto647N-NBs in neurons subjected to sdTIM on a glass-bottom cell culture dish (Step 117A) but can be adapted to the analysis of Atto565-NBs, Alexa647-CTB or other probes. It is imperative to use exactly the same imaging setup for cell-free, fixed-neuron and live-neuron experiments to obtain comparable acquisitions and estimations of the number of internalized ligands in endocytic structures.
-
A
Estimating the number of internalized ligands in fixed neurons
-
i
Prepare a fixative solution of 4% (wt/vol) PFA in PBS by pipetting 0.5 ml of 16% (wt/vol) PFA into 1.5 ml of PBS in a 2-ml Eppendorf tube (see Reagent Setup). Use freshly prepared fixative for best results.
Caution
PFA is hazardous. Use appropriate protective clothing and eyewear, and prepare and handle the solution in a fume hood and dispose of it properly.
-
ii
Repeat Steps 117A(i–vii) and, after the 10-min chase at 37 °C, fix the cells by replacing the medium with 2 ml of 4% (wt/vol) PFA in PBS; allow the cells to incubate for 20 min in a fume hood and then wash them three times with 2 ml of PBS. Continue with Step 125A(iii) immediately.
-
iii
Image internalized VAMP2-pHluorin-bound anti-GFP Atto647N-NBs in fixed neurons using oblique illumination microscopy as described in Step 72.
-
iv
Open the data files obtained in Step 125A(iii) in ImageJ software.
-
v
Follow the Steps 73–78 ('Obtaining emission curves and quantifying the fluorophore-labeling density of the Atto647N-NBs') to calculate the fluorescence decay of internalized ligands with the following modification: analyze the fluorescence decay by expanding the ROI to cover the whole presynapse.
-
vi
The number of simultaneously detected SVs in presynapses at any given time can then be estimated from the number of fluorescence emission steps, the ratio of fluorophore dyes per nanobody and the number of ligands per endocytic structure.
-
i
-
B
Estimating the number of internalized ligands in live neurons
-
i
Repeat Steps 117A(i–viii) and acquire videos of VAMP2-pHluorin fluorescence and VAMP2-pHluorin-bound anti-GFP Atto647N-NB mobility in neurons as described in Steps 72 and 117A(vi).
-
ii
Open the data files obtained in Step B(i) in the ImageJ software.
-
iii
To determine the number of SVs that reside within an active zone at any given time, repeat Step 125A(v and vi). For additional confirmation of the results, continue with Step B(iv).
-
iv
After the single-molecule tracking of VAMP2-pHluorin-bound anti-GFP Atto647N-NBs in live neurons (Steps 117–124), the results obtained in Step B(iii) can be confirmed by recording the fluorescence intensity of each detected spot within the single-particle trajectories.
-
v
Open the 'Spots in Tracks Statistics' text file obtained in Step 123. This file contains the fluorescence intensity assigned to each spot in a track. Plot those against time and count the number of observed steps. The fluorescence level of the SVs trajectories in presynaptic ROIs remains unchanged until either the Atto647N-tag of the internalized nanobody bleaches and the fluorescence intensity trace drops to the background level, or a second SV enters the same diffraction-limited ROI and a steplike increase in fluorescence is detected. The total number of emission steps from the trajectories within the same presynapse reflects the maximum number of SVs that can be detected at any given time.
Critical Step
For optimal results, aim for a maximum of 10 steps. If more steps are detected, decrease the concentration of the ligand or use ligands that contain only one fluorophore. Alternatively, consider prebleaching a subset of the internalized ligands before the images are acquired.
-
i
-
A
Troubleshooting
Troubleshooting advice can be found in Table 1.
Timing
Steps 1–21, photomask fabrication: 5 h 30 min
Step 22, wafer cleaning: 1 h
Steps 23–37, master fabrication: 8 h
Steps 38 and 39, silanization of the master: 2 h
Steps 40–43, soft lithography: 1 h
Steps 44–47, microfluidic device bonding: 1 h
Steps 48–53, sterilization and microfluidic device preparation: 30 min
Steps 54–66, 3D printing of custom-made perfusion lid and preparation of perfusion chamber: 24 h
Steps 67–72, flow-cell preparation and time-lapse imaging of the Atto647N-NBs: 3 h
Steps 73–78, obtaining emission curves and quantifying the fluorophore-labeling density of the Atto647N-NBs: 1–2 h
Steps 79–81, PLL coating for culturing a mix of cortical neurons and astroglial cells: 2 h
Steps 82–92, preparation of NGCM from a mix of cortical neurons and astroglial cells: 2–3 weeks
Steps 93–95, PLL coating of microfluidic devices and glass-bottom dishes: 24 h
Step 96–105, dissection and plating of hippocampal neurons to microfluidic devices or glass-bottom dishes: 2–3 h
Steps 106–108, culture and maintenance of hippocampal neurons: 2–3 weeks
Steps 109–114, primary neuron transfection in microfluidic devices and in glass-bottom cell-culture dishes: 24–48 h
Steps 115–117, induction of synaptic activity and uptake of fluorescently labeled ligands: 30 min–3 h per dish
Steps 118–124, single-molecule tracking with TrackMate: 2 h
Step 125, estimating the number of internalized ligands in endocytic structures: 4 h
Anticipated results
Quantification of labeling density and the number of internalized SVs
sdTIM is a powerful technique for imaging SVs in the crowded presynaptic environment and adjacent axons of cultured hippocampal neurons. The number of Atto647N-fluorophores per nanobody and the number of internalized Atto647N-NBs per SV should be minimized to ensure sparse labeling of the SVs in the presynapses. To quantify the Atto647N-labeling density of the nanobodies, the rate of fluorescence decay of the fluorophores on a glass surface is recorded and the stepwise bleaching is manually counted (Fig. 3a). When using sdTIM to study SV mobility, additional time recordings of the fluorophore emission should be performed at pH 5.4 to mimic the intravesicular acidic pH93. To estimate the number of internalized Atto647N-NBs per SV, hippocampal neurons subjected to sdTIM are fixed and the fluorescence of the internalized Atto647-NBs obtained from the fixed neurons is recorded. The fluorescence decay of the Atto647N-NBs in SVs residing in presynaptic regions can then be manually counted (Fig. 3b). To determine the maximum number of SVs per presynapse that can be detected at any given time point, the fluorescence emission of Atto647N-NBs in live nerve terminals is measured either by counting the number of total emission steps from all trajectories recovered from the presynapses, or by choosing a ROI that covers the entire presynapse. The results obtained allow the assessment of fluorophore-labeling density of the ligands and the estimate of the number of simultaneous detections of SVs at presynapses at any given time. The time course of the fluorescence emission of Atto565-NBs and Alexa647-CTB, or other fluorescently labeled ligands, is determined similarly.
Single-particle tracking in neurons
For single-particle tracking of SVs, hippocampal neurons, grown on glass-bottom cell culture dishes and transfected with VAMP2-pHluorin, are stimulated in high-K+ buffer containing Atto647N-NBs for 5 min (pulse) and chased for 10 min in low-K+ buffer, after which the single-molecule imaging is carried out with oblique illumination microscopy (Fig. 4a). Unquenching of the VAMP2-pHluorin during high-K+ stimulation is followed to discriminate active nerve terminals from the adjacent axonal segments (Box 1; Supplementary Fig. 2a). The increase in fluorescence intensity of VAMP2pHluorin upon high-K+ stimulation can be observed in presynapses but not in axons (Box 1; Supplementary Fig. 2b). The immunolabeling of VAMP2-pHluorin-expressing hippocampal neurons against the endogenous SV protein, synapsin-1, also confirms the identity of active presynapses (Box 1; Supplementary Fig. 3a,b). To ensure the correct localization of internalized ligands, hippocampal neurons expressing VAMP2-pHluorin are subjected to sdTIM, with the exception of using anti-GFP HRP-mCherry-GFP nanotrap (GNT) nanobodies18 and omitting the live-cell imaging of the SVs. After a 5-min pulse and a 10-min chase, neurons are fixed, cytochemically stained and processed for EM as described earlier18. The electron-dense precipitate produced by the HRP substrate can be observed in SVs and, to a lesser extent, in endocytic structures larger than 45 μm in diameter (Supplementary Fig. 3c). In addition, the maturity of the synapses can be assessed by immunolabeling VAMP2-pHluorin-expressing hippocampal neurons against endogenous PSD-95 (Box 1; Supplementary Fig. 3d), thereby labeling the presynaptic and postsynaptic compartments, respectively, and revealing the close connections between the two compartments (Supplementary Fig. 3e,f).
Single-particle tracking of activity-dependent internalization of VAMP2-pHluorin-bound (Fig. 4b) Atto647N-NBs generates super-resolved average intensity maps (Fig. 4c), diffusion coefficient maps (Fig. 4d) and trajectory maps (Fig. 4e) of hippocampal neurons. Related quantification of SV mobility in presynapses and adjacent axons is described by the distribution of Log10D, where D is the diffusion coefficient (Fig. 4f); the mobile to immobile (M/IMM; Fig. 4g) ratio; the mean square displacement (MSD (μm2); Fig. 4h); and the area under the MSD curve (AUC (μm2 s); Fig. 4i). Of note, the same experimental setup can be used in microfluidic devices to image SV mobility at the nerve terminal chamber. The displacement threshold of 0.03 μm2 s−1 for the M/IMM ratio was calculated as described earlier89 (Log10D = 1.45, if [D] = μm2 s−1). In agreement with earlier studies18,25,36,94, the mobility of SVs is greater in axons than in presynapses. For demonstration purposes, we also tracked the VAMP2-pHluorin-bound anti-GFP Atto647N-NBs in the same neuron shown in Fig. 4 with the TrackMate51 platform for ImageJ (Supplementary Fig. 4). As can be appreciated when comparing Figure 4 and Supplementary Figure 4, tracking with the two software packages produced similar results.
Imaging retrograde transport in neurons grown in microfluidic devices
A modification of the pulse–chase sdTIM protocol in neurons grown in microfluidic devices (Fig. 5a) allows investigation of the retrograde transport of Alexa647-CTB and Atto565-NBs. Hippocampal neurons expressing VAMP2-pHluorin (Fig. 5b) are stimulated in the presence of Alexa647-CTB and Atto565-NBs high-K+ buffer for 5 min (pulse) and chased for 2 h in growth medium, after which single-molecule imaging is carried out with oblique illumination microscopy (Fig. 5a). Time-lapse images of retrograde CTB carriers and Atto565-NBs in SVs in an axon passing through the microgrooves of the microfluidic device (Fig. 5c), together with the trajectories of the single molecules of Alexa647-CTB and Atto565-NBs, illustrate retrograde transport toward the cell soma with a characteristic stop-and-go pattern (Fig. 5d). Related quantification of the frequency distribution of Log10D (Fig. 5f), the cumulative frequency distribution (Fig. 5g), the MSD curves (Fig. 5h) and the AUC (Fig. 5i) of CTB carriers and SVs illustrates the differential mobility of the two endocytic compartments. As anticipated based on earlier findings6, CTB carriers, i.e. signaling endosomes, show greater mobility than SVs18, suggesting different mechanisms involved in the transport of the endocytic compartments.
Analyzing the mobility of individual SVs
In addition to addressing the mobility of signaling endosomes at the population level, the mobility of any individual CTB carrier or SV can also be analyzed. Here, as an example, we analyzed an individual Alexa647-CTB containing signaling endosome undergoing characteristic stop-and-go retrograde transport along an axonal segment (Fig. 6a,b; note that the example trajectory is the same as that shown for CTB in Fig. 5e). Measuring the MSD provides a way of assessing whether a particle is undergoing active transport, is caged or is simply undergoing diffusive motion95. The quadratic time dependence of the MSD curve indicates that the CTB carrier is undergoing active transport (Fig. 6c). However, CTB carriers can randomly switch between diffusive and transport states. A recently introduced technique for inferring the parameters underlying heterogeneous motion along a single trajectory combines two powerful mathematical frameworks: HMMs and Bayesian model selection15,16. HMMs are a type of statistical model that assumes that the observations (in this case measurements of particle displacement) are generated from a set of underlying states (e.g., a combination of purely diffusive motion with diffusion constant D1, or active transport with velocity V2 combined with diffusion with diffusion constant D2). Stochastic switching between these states is allowed to occur, and the model determines the sequence of underlying states as a function of time that is most likely to have generated the observations (for instance, pure diffusion with constant D1, followed by transport with constants V2 and D2). However, an HMM does not specify how to choose the assumed number of states (2 in the example above). Assuming the existence of more states would allow the observations to be reproduced more accurately, but at the cost of having a model that is more complex and thus to some extent less powerful. Bayesian model selection provides a rigorous mathematical framework for compromising data fit in favor of model complexity and thereby automatically determining the optimal number of underlying states to best fit the data. Combined with HMMs, this provides a highly effective method for determining both (i) the number of active transport and diffusive states underlying a particular particle trajectory, and (ii) when transitions between these states occur. This technique can be used to analyze any individual trajectory or a set of pooled trajectories to infer motion parameters. We analyzed sets of pooled SV trajectories using parallel computing (with nodal specification of 512 GB of main memory, two Intel Xeon E5v4 processors, providing 72 threads of execution, two NVidia K80 GPUs, a 250 TB parallel file system (General Parallel File System (GPFS)) and 100 GbE Interconnect for storage input/output)18, which is recommended when analyzing large sets of pooled trajectories15.
When we analyzed the trajectory shown in Figure 6a with a maximum of three states using HMM–Bayes software (http://hmm-bayes.org/), we found that a two-state model with one purely diffusive state and one retrograde transport state provided the best fit, and that the CTB carrier switched between diffusive and transport states during its motion along the axon (Fig. 6d,e). Localization precision of 30 nm, estimated from the purely diffusive segment of the trajectory (Supplementary Fig. 5a,b), was used to correct the diffusion coefficients. Distributions of Alexa647-CTB and Atto565-NB localization precisions in hippocampal neurons are shown in Supplementary Figure 5c,d, respectively.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Lemke, E.A. & Klingauf, J. Single synaptic vesicle tracking in individual hippocampal boutons at rest and during synaptic activity. J. Neurosci. 25, 11034–11044 (2005).
Shtrahman, M., Yeung, C., Nauen, D.W., Bi, G.Q. & Wu, X.L. Probing vesicle dynamics in single hippocampal synapses. Biophys. J. 89, 3615–3627 (2005).
Westphal, V. et al. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science 320, 246–249 (2008).
Giannone, G., Hosy, E., Sibarita, J.B., Choquet, D. & Cognet, L. High-content super-resolution imaging of live cell by uPAINT. Methods Mol. Biol. 950, 95–110 (2013).
Bademosi, A.T. et al. In vivo single-molecule imaging of syntaxin1A reveals polyphosphoinositide- and activity-dependent trapping in presynaptic nanoclusters. Nat. Commun. 8, 13660 (2017).
Wang, T. et al. Flux of signalling endosomes undergoing axonal retrograde transport is encoded by presynaptic activity and TrkB. Nat. Commun. 7, 12976 (2016).
Wang, T. et al. Control of autophagosome axonal retrograde flux by presynaptic activity unveiled using botulinum neurotoxin type a. J. Neurosci. 35, 6179–6194 (2015).
Bal, M. et al. Reelin mobilizes a VAMP7-dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission. Neuron 80, 934–946 (2013).
Chung, C., Barylko, B., Leitz, J., Liu, X. & Kavalali, E.T. Acute dynamin inhibition dissects synaptic vesicle recycling pathways that drive spontaneous and evoked neurotransmission. J. Neurosci. 30, 1363–1376 (2010).
Fredj, N.B. & Burrone, J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat. Neurosci. 12, 751–758 (2009).
Hua, Z. et al. v-SNARE composition distinguishes synaptic vesicle pools. Neuron 71, 474–487 (2011).
Raingo, J. et al. VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission. Nat. Neurosci. 15, 738–745 (2012).
Sara, Y., Virmani, T., Deak, F., Liu, X. & Kavalali, E.T. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron 45, 563–573 (2005).
Guillaud, L., Dimitrov, D. & Takahashi, T. Presynaptic morphology and vesicular composition determine vesicle dynamics in mouse central synapses. Elife 6 http://dx.doi.org/10.7554/eLife.24845 (2017).
Monnier, N. et al. Inferring transient particle transport dynamics in live cells. Nat. Methods 12, 838–840 (2015).
Persson, F., Linden, M., Unoson, C. & Elf, J. Extracting intracellular diffusive states and transition rates from single-molecule tracking data. Nat. Methods 10, 265–269 (2013).
Harper, C.B. et al. Botulinum neurotoxin type-A enters a non-recycling pool of synaptic vesicles. Sci. Rep. 6, 19654 (2016).
Joensuu, M. et al. Subdiffractional tracking of internalized molecules reveals heterogeneous motion states of synaptic vesicles. J. Cell Biol. 215, 277–292 (2016).
Fiolka, R. Clearer view for TIRF and oblique illumination microscopy. Opt. Express 24, 29556–29567 (2016).
Giannone, G. et al. Dynamic superresolution imaging of endogenous proteins on living cells at ultra-high density. Biophys. J. 99, 1303–1310 (2010).
Tokunaga, M., Imamoto, N. & Sakata-Sogawa, K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat. Methods 5, 159–161 (2008).
Miesenbock, G., De Angelis, D.A. & Rothman, J.E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195 (1998).
Pellizzari, R., Rossetto, O., Schiavo, G. & Montecucco, C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354, 259–268 (1999).
Esposito, G., Ana Clara, F. & Verstreken, P. Synaptic vesicle trafficking and Parkinson's disease. Dev. Neurobiol. 72, 134–144 (2012).
Kamin, D. et al. High- and low-mobility stages in the synaptic vesicle cycle. Biophys. J. 99, 675–684 (2010).
Hua, Y. et al. A readily retrievable pool of synaptic vesicles. Nat. Neurosci. 14, 833–839 (2011).
Gimber, N., Tadeus, G., Maritzen, T., Schmoranzer, J. & Haucke, V. Diffusional spread and confinement of newly exocytosed synaptic vesicle proteins. Nat. Commun. 6, 8392 (2015).
Cui, B. et al. One at a time, live tracking of NGF axonal transport using quantum dots. Proc. Natl. Acad. Sci. USA 104, 13666–13671 (2007).
Zhang, K. et al. Single-molecule imaging of NGF axonal transport in microfluidic devices. Lab. Chip 10, 2566–2573 (2010).
Manley, S. et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 5, 155–157 (2008).
Aad, G. et al. Two-particle Bose-Einstein correlations in collisions at [Formula: see text] 0.9 and 7 TeV measured with the ATLAS detector. Eur. Phys. J C Part Fields 75, 466 (2015).
Maschi, D. & Klyachko, V.A. Spatiotemporal regulation of synaptic vesicle fusion sites in central synapses. Neuron 94, 65–73.e3 (2017).
Chang, Y.P., Pinaud, F., Antelman, J. & Weiss, S. Tracking bio-molecules in live cells using quantum dots. J. Biophotonics 1, 287–298 (2008).
Gordon, S.L., Leube, R.E. & Cousin, M.A. Synaptophysin is required for synaptobrevin retrieval during synaptic vesicle endocytosis. J. Neurosci. 31, 14032–14036 (2011).
Gordon, S.L., Harper, C.B., Smillie, K.J. & Cousin, M.A. A fine balance of synaptophysin levels underlies efficient retrieval of synaptobrevin II to synaptic vesicles. PLoS One 11, e0149457 (2016).
Lee, S., Jung, K.J., Jung, H.S. & Chang, S. Dynamics of multiple trafficking behaviors of individual synaptic vesicles revealed by quantum-dot based presynaptic probe. PLoS One 7, e38045 (2012).
Pavani, S.R.P. et al. Three-dimensional, single-molecule fluorescence imaging beyond the diffraction limit by using a double-helix point spread function. Proc. Natl. Acad. Sci. USA 106, 2995–2999 (2009).
Osseforth, C., Moffitt, J.R., Schermelleh, L. & Michaelis, J. Simultaneous dual-color 3D STED microscopy. Opt. Express 22, 7028–7039 (2014).
Wildanger, D., Medda, R., Kastrup, L. & Hell, S.W. A compact STED microscope providing 3D nanoscale resolution. J. Microsc. 236, 35–43 (2009).
Huang, B., Wang, W., Bates, M. & Zhuang, X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319, 810–813 (2008).
Hayashi, S. & Okada, Y. Ultrafast superresolution fluorescence imaging with spinning disk confocal microscope optics. Mol. Biol. Cell 26, 1743–1751 (2015).
Azuma, T. & Kei, T. Super-resolution spinning-disk confocal microscopy using optical photon reassignment. Opt. Express 23, 15003–15011 (2015).
York, A.G. et al. Instant super-resolution imaging in live cells and embryos via analog image processing. Nat. Methods 10, 1122–1126 (2013).
Gustafsson, N. et al. Fast live-cell conventional fluorophore nanoscopy with ImageJ through super-resolution radial fluctuations. Nat. Commun. 7, 12471 (2016).
Li, D. et al. Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics. Science 349, aab3500 (2015).
Chenouard, N. et al. Objective comparison of particle tracking methods. Nat. Methods 11, 281–289 (2014).
Izeddin, I. et al. Wavelet analysis for single molecule localization microscopy. Opt. Express 20, 2081–2095 (2012).
Racine, V. et al. Multiple-target tracking of 3D fluorescent objects based on simulated annealing. 3rd IEEE International Symposium on Biomedical Imaging: Macro to Nano, Vols 1-3 1020–1023 (2006).
Kechkar, A., Nair, D., Heilemann, M., Choquet, D. & Sibarita, J.B. Real-time analysis and visualization for single-molecule based super-resolution microscopy. PLoS One 8, e62918 (2013).
Nair, D. et al. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J. Neurosci. 33, 13204–13224 (2013).
Tinevez, J.Y. et al. TrackMate: an open and extensible platform for single-particle tracking. Methods 115, 80–90 (2017).
Cosker, K.E. & Segal, R.A. Neuronal signaling through endocytosis. Cold Spring Harb. Perspect. Biol. 6,a020669 (2014).
Yamashita, N. & Kuruvilla, R. Neurotrophin signaling endosomes: biogenesis, regulation, and functions. Curr. Opin. Neurobiol. 39, 139–145 (2016).
Ito, K. & Enomoto, H. Retrograde transport of neurotrophic factor signaling: implications in neuronal development and pathogenesis. J. Biochem. 160, 77–85 (2016).
Beck, A., Goetsch, L., Dumontet, C. & Corvaia, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 16, 315–337 (2017).
Copolovici, D.M., Langel, K., Eriste, E. & Langel, U. Cell-penetrating peptides: design, synthesis, and applications. ACS Nano 8, 1972–1994 (2014).
Raucher, D. & Ryu, J.S. Cell-penetrating peptides: strategies for anticancer treatment. Trends Mol. Med. 21, 560–570 (2015).
Yamauchi, Y. & Helenius, A. Virus entry at a glance. J. Cell Sci. 126, 1289–1295 (2013).
Grove, J. & Marsh, M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 195, 1071–1082 (2011).
Koyuncu, O.O., Hogue, I.B. & Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 13, 379–393 (2013).
Harper, C.B., Popoff, M.R., McCluskey, A., Robinson, P.J. & Meunier, F.A. Targeting membrane trafficking in infection prophylaxis: dynamin inhibitors. Trends Cell Biol. 23, 90–101 (2013).
Pizarro-Cerda, J. & Cossart, P. Bacterial adhesion and entry into host cells. Cell 124, 715–727 (2006).
Doherty, G.J. & McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 (2009).
Grant, B.D. & Donaldson, J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597–608 (2009).
Sorkin, A. & von Zastrow, M. Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609–622 (2009).
Skinner, M. Endocytosis: curvature proteins direct traffic. Nat. Rev. Mol. Cell Biol. 11, 466 (2010).
Soldati, T. & Schliwa, M. Powering membrane traffic in endocytosis and recycling. Nat. Rev. Mol. Cell Biol. 7, 897–908 (2006).
Johannes, L., Parton, R.G., Bassereau, P. & Mayor, S. Building endocytic pits without clathrin. Nat. Rev. Mol. Cell Biol. 16, 311–321 (2015).
Vanhauwaert, R. et al. The SAC1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals. EMBO J. 36, 1392–1411 (2017).
Barrangou, R. & Doudna, J.A. Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 34, 933–941 (2016).
Cullen, P.J. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat. Rev. Mol. Cell Biol. 9, 574–582 (2008).
Rottner, K., Hanisch, J. & Campellone, K.G. WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol. 20, 650–661 (2010).
Gallon, M. & Cullen, P.J. Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 43, 33–47 (2015).
Puthenveedu, M.A. et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 143, 761–773 (2010).
van Weering, J.R., Verkade, P. & Cullen, P.J. SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation. Traffic 13, 94–107 (2012).
Rizopoulos, Z. et al. Vaccinia virus infection requires maturation of macropinosomes. Traffic 16, 814–831 (2015).
Ketel, K. et al. A phosphoinositide conversion mechanism for exit from endosomes. Nature 529, 408–412 (2016).
Ramage, H. & Cherry, S. Virus-host interactions: from unbiased genetic screens to function. Annu. Rev. Virol. 2, 497–524 (2015).
Sun, E., He, J. & Zhuang, X. Live cell imaging of viral entry. Curr. Opin. Virol. 3, 34–43 (2013).
Brandenburg, B. & Zhuang, X. Virus trafficking - learning from single-virus tracking. Nat. Rev. Microbiol. 5, 197–208 (2007).
Grove, J. Super-resolution microscopy: a virus' eye view of the cell. Viruses 6, 1365–1378 (2014).
Gray, R.D. et al. VirusMapper: open-source nanoscale mapping of viral architecture through super-resolution microscopy. Sci. Rep. 6, 29132 (2016).
Laine, R.F. et al. Structural analysis of herpes simplex virus by optical super-resolution imaging. Nat. Commun. 6, 5980 (2015).
Muller, B. & Heilemann, M. Shedding new light on viruses: super-resolution microscopy for studying human immunodeficiency virus. Trends Microbiol. 21, 522–533 (2013).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Schindelin, J., Rueden, C.T., Hiner, M.C. & Eliceiri, K.W. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol. Reprod. Dev. 82, 518–529 (2015).
Arena, E.T., Tinevez, J.Y., Nigro, G., Sansonetti, P.J. & Marteyn, B.S. The infectious hypoxia: occurrence and causes during Shigella infection. Microbes Infect. 19, 157–165 (2017).
Durisic, N., Cuervo, L.L. & Lakadamyali, M. Quantitative super-resolution microscopy: pitfalls and strategies for image analysis. Curr. Opin. Chem. Biol. 20, 22–28 (2014).
Constals, A. et al. Glutamate-induced AMPA receptor desensitization increases their mobility and modulates short-term plasticity through unbinding from Stargazin. Neuron 85, 787–803 (2015).
Durisic, N., Laparra-Cuervo, L., Sandoval-Alvarez, A., Borbely, J.S. & Lakadamyali, M. Single-molecule evaluation of fluorescent protein photoactivation efficiency using an in vivo nanotemplate. Nat. Methods 11, 156–162 (2014).
Brown, W.J. & Farquhar, M.G. Immunoperoxidase methods for the localization of antigens in cultured cells and tissue sections by electron microscopy. Methods Cell Biol. 31, 553–569 (1989).
Harper, C.B. et al. Dynamin inhibition blocks botulinum neurotoxin type A endocytosis in neurons and delays botulism. J. Biol. Chem. 286, 35966–35976 (2011).
Mel'nik, V.I., Glebov, R.N. & Kryzhanovskii, G.N. [ATP-dependent proton translocation across the synaptic vesicle membrane in the brain of rats]. Biull. Eksp. Biol. Med. 99, 35–38 (1985).
Sankaranarayanan, S. & Ryan, T.A. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat. Cell Biol. 2, 197–204 (2000).
Ruthardt, N., Lamb, D.C. & Brauchle, C. Single-particle tracking as a quantitative microscopy-based approach to unravel cell entry mechanisms of viruses and pharmaceutical nanoparticles. Mol. Ther. 19, 1199–1211 (2011).
Rizzoli, S.O. & Betz, W.J. The structural organization of the readily releasable pool of synaptic vesicles. Science 303, 2037–2039 (2004).
Dempsey, G.T., Vaughan, J.C., Chen, K.H., Bates, M. & Zhuang, X. Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nat. Methods 8, 1027–1036 (2011).
Chamma, I., Rossier, O., Giannone, G., Thoumine, O. & Sainlos, M. Optimized labeling of membrane proteins for applications to super-resolution imaging in confined cellular environments using monomeric streptavidin. Nat. Protoc. 12, 748–763 (2017).
Wilhelm, B.G. et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344, 1023–1028 (2014).
Fernandez-Suarez, M. & Ting, A.Y. Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. Mol. Cell Biol. 9, 929–943 (2008).
Acknowledgements
The super-resolution imaging was carried out at the Queensland Brain Institute's (QBI's) Advanced Microimaging and Analysis Facility with the help of A. Chien. We thank R.G. Parton (Institute for Molecular Bioscience, The University of Queensland, Australia) for helpful comments, N. Valmas (QBI) for the photography and the schematic illustration in Figure 1, J. Carroll and his team (QBI) for research computing infrastructure support, data management and processing, and R. Tweedale (QBI) for critical appraisal of the manuscript. We also thank J.B. Sibarita (Centre National de la Recherche Scientifique, Interdisciplinary Institute for Neuroscience, University of Bordeaux. France) for helpful comments and help with the PalmTracer software. We thank T. Wang and A. Papadopoulos for performing the original studies. This work was supported by an Australian Research Council Discovery Project grant (DP150100539) an Australian Research Council Linkage Infrastructure, Equipment, and Facilities grant (LE130100078) and a National Health and Medical Research Council (NHMRC) grant (APP1120381) to F.A.M. M.J. is supported by an Academy of Finland Postdoctoral Research Fellowship (298124), G.B. by the Finnish Cancer Foundation, P.P. and N.D. by University of Queensland Postdoctoral Research Fellowships and N.D. by a UQ Early Career Researcher Grant (UQECR1718789). R.M.-M. is funded by the Clem Jones Centre for Ageing Dementia Research. F.A.M. is a Senior Research Fellow of the National Health and Medical Research Council (1060075).
Author information
Authors and Affiliations
Contributions
M.J. and R.M.-M. performed the experiments and analyzed the data. M.J. prepared the figures and wrote the paper with R.M.-M., P.P., N.D., R.A., G.B. and F.A.M. N.R.G. prepared the microfluidic devices and wrote the description of their preparation, which was supervised by J.J.C.-W. The perfusion lid was designed by M.P. and M.J. and was constructed by M.P. M.J. performed the nanobody fluorescence intensity analysis with N.D. P.P. performed the Bayesian model selection to HMM analysis, which was supervised by G.J.G. N.D. and P.P. estimated the localization precision. N.D. performed the single-molecule tracking with TrackMate. M.J., M.M. and G.B. prepared the schematic in Figure 2, and Supplementary Table 1. F.A.M. designed and supervised the studies. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Custom-made perfusion lid for glass-bottom cell-culture dishes.
(a) The perfusion lid has supported inlet and outlet holes located at the rim of the lid, thereby enabling liquid exchange on top of the plastic portion of the cell culture dish. This allows gentle injection and aspiration of liquid to and from the culture dish, without disturbing the neurons grown on the glass-bottom part of the dish. (b) The lid has 4 spacers to allow gas exchange between the culture dish and the microscope chamber. (c) A photograph of the perfusion lid with the glass-bottom cell culture dish. Note that the 2 mm aspiration tube going through the lid hole needs to be placed at a height where it is in close proximity to the plastic bottom of the culture dish (open arrow) to allow efficient aspiration of the buffer. The injection tubing can be placed further away from the dish bottom (arrow). (d) The perfusion chamber is secured tightly with clamps in the microscope chamber and (e) the injection and aspiration syringes are attached to the 2 mm tubes with blunt-end needles which can be changed when needed.
Supplementary Figure 2 Monitoring synaptic vesicle exocytic events with VAMP2-pHluorin to discriminate active presynapses from adjacent axons.
(a) A mosaic image of rat E18 hippocampal neurons cultured in a microfluidic device, and transfected with VAMP2-pHluorin on DIV14 and imaged on DIV16. Neurons are stimulated in high K+ (both terminal and soma wells) and the fluorescence of VAMP2-pHluorin is recorded in the nerve terminal chamber. (b and c) Quantification of VAMP2-pHluorin fluorescence of the hippocampal neuron shown in (a). The graphs show the measured fluorescence intensity for a representative (b) presynapse (boxed area in a) and (c) axonal segment (dashed box in a), in low K+ (i.e. resting) and high K+ (i.e. stimulated) conditions, demonstrating an increase in the average fluorescence of the VAMP2-pHluorin (i.e. unquenching) in the presynapse upon release and exposure to the extracellular pH. Values presented in the graphs (b and c) show mean fluorescence after stimulation (high K+) normalized to the mean fluorescence in the resting neuron prior stimulation, i.e. in low K+. The values are shown as an example from n = 1 hippocampal neuron. Please see Box 1 for more details. Bar 10 μm. All the experiments were carried out in accordance with relevant institutional and governmental ethical guidelines and regulations (Animal Ethics Approval QBI/254/16/NHMRC).
Supplementary Figure 3 Assessing synaptic identity, functionality and maturity.
(a) Cultured rat E18 hippocampal neurons are transfected with VAMP2-pHluorin (green) on DIV14, fixed on DIV16 and immunolabeled against endogenous Synapsin-1 (magenta). The boxed area in (a) is shown at higher magnification in (b) demonstrating the co-localization of the two synaptic markers in presynapses (arrowhead). (c) To assess synaptic functionality and correct localization of internalized nanobodies in recycling SVs, VAMP2-pHluorin-transfected neurons are subjected to sdTIM using HRP-tagged mCherry-GNT. After fixation and cytochemical staining, neurons are processed for electron microscopy. HRP-precipitate in SVs (open arrowheads) and unstained vesicles (arrowheads) are indicated. To assess the maturity of synapses in the terminal well of the microfluidic devices (d) VAMP2-pHluorin (green)-expressing neurons in the soma well are fixed and immunolabeled against endogenous PSD-95 (magenta) to identify dendrites in the terminal well. (e) Confocal image of the VAMP2-pHluorin-positive axon passing through a microfluidic channel and forming synaptic connections with the neurons cultured in the terminal well. (f) Higher magnification images from the boxed area in (e) show the punctate staining pattern of postsynaptic density (arrowhead) co-localizing with and adjacent to presynaptic VAMP2-pHluorin (open arrowhead). Bars are 5 μm (a and e), 0.1 μm (c), 100 μm (d) and 1 μm (b and f). See Box 2 for more details. All the experiments were carried out in accordance with relevant institutional and governmental ethical guidelines and regulations (Animal Ethics Approval QBI/254/16/NHMRC).
Supplementary Figure 4 Single-molecule tracking with TrackMate.
Quantification of the SV mobility in presynapses (Ps) and axons (Ax) using the TrackMate plug-in for ImageJ. The graphs show (a) the frequency distribution of the average Log10 diffusion coefficient (D) and (b) MSD [μm2]. The average numbers ±SEM are obtained from presynapses (n = 6 presynapses from where 1,000 trajectories) and axonal segments (n = 12 axonal segments from where 3,600) of the same neuron presented in Fig. 4. All the experiments were carried out in accordance with relevant institutional and governmental ethical guidelines and regulations (Animal Ethics Approval QBI/254/16/NHMRC).
Supplementary Figure 5 Estimation of localization precision using two methods.
(a-b) localization precision was estimated from the MSD curve. (a) A purely diffusive segment of the CTB trajectory shown in Fig. 6d was employed to estimate the localization precision (σ). (b) MSD [μm2] curve of the trajectory segment shown in (a). The MSD curve was fitted to the equation MSD(τ) = 4σ2 + 4Dτ to estimate the localization precision, where D is the diffusion coefficient and τ is the time lag. The estimated σ (30 nm) was used to correct the diffusion coefficients estimated using HMM-Bayes analysis in Fig. 6. (c-d) Localization precision was estimated by fitting each fluorescent spot to a Gaussian distribution and localization precision was approximated as s/√N, where s is the standard deviation of the Gaussian function and N is the number of photons captured from a fluorescent spot. Distributions of localization precision of (c) Alexa647-CTB (~500 localizations) and (d) Atto565-NBs (>21,000 localizations) in hippocampal neurons. The average localization precision for Alexa647-CTB was 43 ± 9 nm and 32 ± 4 nm for Atto565-NBs. All the experiments were carried out in accordance with relevant institutional and governmental ethical guidelines and regulations (Animal Ethics Approval QBI/254/16/NHMRC).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Table 1. (PDF 1299 kb)
Supplementary Data 1–3
Supplementary Data 1. Description of steps for single-molecule tracking with TrackMate and MATLAB routine. Supplementary Data 2. 3D-print design. Supplementary Data 3. mask.gds file. (ZIP 5539 kb)
Retrograde transport of CTB.
Hippocampal neurons cultured in microfluidic devices are stimulated for 5 min in high K+ in the presence of Alexa-647-CTB (50 ng μ−1) applied in the nerve terminal chamber. Following a 2-h chase, single Alexa-647-CTB molecules undergoing retrograde transport (left to right) can be detected in the axons that pass through the microfluidic channels. Note that a few molecules also undergo anterograde transport (right to left). Time-lapse imaging was carried out at 50 Hz and 20 ms exposure at +37 °C on a microscope equipped with an iLas2 double-laser illuminator, a CFI Apo TIRF 100× 1.49 NA objective and an Evolve512 delta EMCCD camera. Image acquisition was performed using Metamorph software. For presentation purposes, 2,500-frame acquisition is presented. Playback = 50 frames s−1. Scale bar, 5 μm. Imaging was carried out in accordance with relevant institutional and governmental ethical guidelines and regulations (Animal Ethics Approval QBI/254/16/NHMRC). (AVI 307347 kb)
Rights and permissions
About this article
Cite this article
Joensuu, M., Martínez-Mármol, R., Padmanabhan, P. et al. Visualizing endocytic recycling and trafficking in live neurons by subdiffractional tracking of internalized molecules. Nat Protoc 12, 2590–2622 (2017). https://doi.org/10.1038/nprot.2017.116
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.116
This article is cited by
-
Synapsin 2a tetramerisation selectively controls the presynaptic nanoscale organisation of reserve synaptic vesicles
Nature Communications (2024)
-
Super-resolved trajectory-derived nanoclustering analysis using spatiotemporal indexing
Nature Communications (2023)
-
Fyn nanoclustering requires switching to an open conformation and is enhanced by FTLD-Tau biomolecular condensates
Molecular Psychiatry (2023)
-
Tau forms synaptic nano-biomolecular condensates controlling the dynamic clustering of recycling synaptic vesicles
Nature Communications (2023)
-
Fear extinction is regulated by the activity of long noncoding RNAs at the synapse
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.