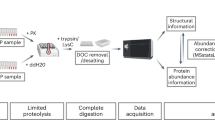
Abstract
Protein structural changes induced by external perturbations or internal cues can profoundly influence protein activity and thus modulate cellular physiology. A number of biophysical approaches are available to probe protein structural changes, but these are not applicable to a whole proteome in a biological extract. Limited proteolysis-coupled mass spectrometry (LiP-MS) is a recently developed proteomics approach that enables the identification of protein structural changes directly in their complex biological context on a proteome-wide scale. After perturbations of interest, proteome extracts are subjected to a double-protease digestion step with a nonspecific protease applied under native conditions, followed by complete digestion with the sequence-specific protease trypsin under denaturing conditions. This sequential treatment generates structure-specific peptides amenable to bottom-up MS analysis. Next, a proteomics workflow involving shotgun or targeted MS and label-free quantification is applied to measure structure-dependent proteolytic patterns directly in the proteome extract. Possible applications of LiP-MS include discovery of perturbation-induced protein structural alterations, identification of drug targets, detection of disease-associated protein structural states, and analysis of protein aggregates directly in biological samples. The approach also enables identification of the specific protein regions involved in the structural transition or affected by the binding event. Sample preparation takes approximately 2 d, followed by one to several days of MS and data analysis time, depending on the number of samples analyzed. Scientists with basic biochemistry training can implement the sample preparation steps. MS measurement and data analysis require a background in proteomics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Walther, T.C. & Mann, M. Mass spectrometry-based proteomics in cell biology. J. Cell Biol. 190, 491–500 (2010).
Feng, Y. et al. Global analysis of protein structural changes in complex proteomes. Nat. Biotechnol. 32, 1036–1044 (2014).
Leuenberger, P. et al. Cell-wide analysis of protein thermal unfolding reveals determinants of thermostability. Science 355, eaai7825 (2017).
Stauffer, S. et al. Stepwise priming by acidic pH and a high K+ concentration is required for efficient uncoating of influenza A virus cores after penetration. J. Virol. 88, 13029–13046 (2014).
Geiger, R. et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842 (2016).
Aceto, A. et al. Analysis by limited proteolysis of domain organization and GSH-site arrangement of bacterial glutathione transferase B1-1. Int. J. Biochem. Cell Biol. 27, 1033–1041 (1995).
Dieckmann, R. et al. Probing the domain structure and ligand-induced conformational changes by limited proteolysis of tyrocidine synthetase 1. J. Mol. Biol. 288, 129–140 (1999).
Nouwen, N. et al. Domain structure of secretin PulD revealed by limited proteolysis and electron microscopy. EMBO J. 19, 2229–2236 (2000).
Pagnozzi, D. et al. Stoichiometry and topology of the complex of the endogenous ATP synthase inhibitor protein IF(1) with calmodulin. Biochemistry 49, 7542–7552 (2010).
Peng, Z.Y. & Kim, P.S. A protein dissection study of a molten globule. Biochemistry 33, 2136–2141 (1994).
Polverino de Laureto, P. et al. Protein aggregation and amyloid fibril formation by an SH3 domain probed by limited proteolysis. J. Mol. Biol. 334, 129–141 (2003).
Eftink, M.R. Fluorescence techniques for studying protein structure. Methods Biochem. Anal. 35, 127–205 (1991).
Ilari, A. & Savino, C. Protein structure determination by x-ray crystallography. Methods Mol. Biol. 452, 63–87 (2008).
Pelton, J.T. & McLean, L.R. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 277, 167–176 (2000).
Konermann, L., Pan, J. & Liu, Y.H. Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chem. Soc. Rev. 40, 1224–1234 (2011).
Heyduk, T. Measuring protein conformational changes by FRET/LRET. Curr. Opin. Biotechnol. 13, 292–296 (2002).
Sakakibara, D. et al. Protein structure determination in living cells by in-cell NMR spectroscopy. Nature 458, 102–105 (2009).
Herzog, F. et al. Structural probing of a protein phosphatase 2A network by chemical cross-linking and mass spectrometry. Science 337, 1348–1352 (2012).
Roeser, J. et al. Oxidative protein labeling in mass-spectrometry-based proteomics. Anal. Bioanal. Chem. 397, 3441–3455 (2010).
Mendoza, V.L. & Vachet, R.W. Probing protein structure by amino acid-specific covalent labeling and mass spectrometry. Mass Spectrom. Rev. 28, 785–815 (2009).
Picotti, P. & Aebersold, R. Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nat. Methods 9, 555–566 (2012).
Liu, F. et al. Proteome-wide profiling of protein assemblies by cross-linking mass spectrometry. Nat. Methods 12, 1179–1184 (2015).
Kosinski, J. et al. Molecular architecture of the inner ring scaffold of the human nuclear pore complex. Science 352, 363–365 (2016).
Leitner, A., Walzthoeni, T. & Aebersold, R. Lysine-specific chemical cross-linking of protein complexes and identification of cross-linking sites using LC-MS/MS and the xQuest/xProphet software pipeline. Nat. Protoc. 9, 120–137 (2014).
Savitski, M.M. et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 346, 1255784 (2014).
Fontana, A. et al. Probing protein structure by limited proteolysis. Acta Biochim. Pol. 51, 299–321 (2004).
Monti, M. & Pucci, P. Limited proteolysis mass spectrometry of protein complexes. Mass Spectrometry of Protein Interactions (ed. Downard, K.) Ch. 4 (Wiley, 2007).
Polverino de Laureto, P. et al. Probing the structure of human growth hormone by limited proteolysis. Int. J. Pept. Protein Res. 45, 200–208 (1995).
Picotti, P. et al. Modulation of the structural integrity of helix F in apomyoglobin by single amino acid replacements. Protein Sci. 13, 1572–1585 (2004).
Glatter, T. et al. Large-scale quantitative assessment of different in-solution protein digestion protocols reveals superior cleavage efficiency of tandem Lys-C/trypsin proteolysis over trypsin digestion. J. Proteome Res. 11, 5145–5156 (2012).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Rost, H.L. et al. OpenMS: a flexible open-source software platform for mass spectrometry data analysis. Nat. Methods 13, 741–748 (2016).
Perez-Riverol, Y. et al. Open source libraries and frameworks for mass spectrometry based proteomics: a developer's perspective. Biochim. Biophys. Acta 1844, 63–76 (2014).
Feng, Y. & Picotti, P. Selected reaction monitoring to measure proteins of interest in complex samples: a practical guide. Methods Mol. Biol. 1394, 43–56 (2016).
MacLean, B. et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968 (2010).
Choi, M. et al. MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics 30, 2524–2526 (2014).
Moulder, R., Goo, Y.A. & Goodlett, D.R. Label-free quantitation for clinical proteomics. Methods Mol. Biol. 1410, 65–76 (2016).
Richards, A.L. et al. One-hour proteome analysis in yeast. Nat. Protoc. 10, 701–714 (2015).
Surinova, S. et al. Automated selected reaction monitoring data analysis workflow for large-scale targeted proteomic studies. Nat. Protoc. 8, 1602–1619 (2013).
Wyss, M. et al. Limited proteolysis of creatine kinase. Implications for three-dimensional structure and for conformational substrates. Biochemistry 32, 10727–10735 (1993).
Vita, C., Dalzoppo, D. & Fontana, A. Limited proteolysis of thermolysin by subtilisin: isolation and characterization of a partially active enzyme derivative. Biochemistry 24, 1798–1806 (1985).
Martini, F. et al. Investigation of intra-domain and inter-domain interactions of glutathione transferase P1-1 by limited chymotryptic cleavage. Eur. J. Biochem. 218, 845–851 (1993).
Hatori, Y. et al. Domain organization and movements in heavy metal ion pumps: papain digestion of CopA, a Cu+-transporting ATPase. J. Biol. Chem. 282, 25213–25221 (2007).
Griese, M. et al. Limited proteolysis of surfactant protein D causes a loss of its calcium-dependent lectin functions. Biochim. Biophys. Acta 1638, 157–163 (2003).
Scolaro, L.A., Mersich, S.E. & Damonte, E.B. A mouse attenuated mutant of Junin virus with an altered envelope glycoprotein. Arch. Virol. 111, 257–362 (1990).
Kordyukova, L.V. et al. Influenza A virus M1 protein structure probed by in situ limited proteolysis with bromelain. Protein Pept. Lett. 15, 922–930 (2008).
Polverino de Laureto, P. et al. Limited proteolysis of bovine alpha-lactalbumin: isolation and characterization of protein domains. Protein Sci. 8, 2290–2303 (1999).
Kim, J. et al. Characterization of a unique IgG1 mAb CEX profile by limited Lys-C proteolysis/CEX separation coupled with mass spectrometry and structural analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878, 1973–1981 (2010).
Laganowsky, A. et al. Mass spectrometry of intact membrane protein complexes. Nat. Protoc. 8, 639–651 (2013).
Becher, I. et al. Chemoproteomics reveals time-dependent binding of histone deacetylase inhibitors to endogenous repressor complexes. ACS Chem. Biol. 9, 1736–1746 (2014).
Cadene, M. & Chait, B.T. A robust, detergent-friendly method for mass spectrometric analysis of integral membrane proteins. Anal. Chem. 72, 5655–5658 (2000).
Proc, J.L. et al. A quantitative study of the effects of chaotropic agents, surfactants, and solvents on the digestion efficiency of human plasma proteins by trypsin. J. Proteome Res. 9, 5422–5437 (2010).
Stark, G.R., Stein, W.H. & Moore, S. Reactions of the cyanate present in aqueous urea with amino acids and proteins. J. Biol. Chem. 235, 3177–3181 (1960).
Kang, S.C. et al. Guanidine hydrochloride extraction and detection of prion proteins in mouse and hamster prion diseases by ELISA. J. Pathol. 199, 534–541 (2003).
Barron, R.M. et al. High titers of transmissible spongiform encephalopathy infectivity associated with extremely low levels of PrPSc in vivo. J. Biol. Chem. 282, 35878–35886 (2007).
Meyer, R.K. et al. Detection of bovine spongiform encephalopathy-specific PrP(Sc) by treatment with heat and guanidine thiocyanate. J. Virol. 73, 9386–9392 (1999).
Ren, D. et al. An improved trypsin digestion method minimizes digestion-induced modifications on proteins. Anal. Biochem. 392, 12–21 (2009).
Rashid, F., Sharma, S. & Bano, B. Comparison of guanidine hydrochloride (GdnHCl) and urea denaturation on inactivation and unfolding of human placental cystatin (HPC). Protein J. 24, 283–292 (2005).
Michalski, A., Cox, J. & Mann, M. More than 100,000 detectable peptide species elute in single shotgun proteomics runs but the majority is inaccessible to data-dependent LC-MS/MS. J. Proteome Res. 10, 1785–1793 (2011).
Biniossek, M.L. & Schilling, O. Enhanced identification of peptides lacking basic residues by LC-ESI-MS/MS analysis of singly charged peptides. Proteomics 12, 1303–1309 (2012).
Acknowledgements
ACKNOWLEDGMENTS
We thank O. Schubert (UCLA) for insightful discussions. We also thank T. Lehmann for building the device to hold the sample tubes in the water bath and G. de Franceschi for contributing to the setup of the original LiP-MS protocol. P.P. is supported by an EU FP7-ERC Starting Grant (FP7-ERC-StG-337965), a 'Foerderungsprofessur' grant from the Swiss National Science Foundation (grant PP00P3_133670), and Promedica Stiftung (grant 2-70669-11). Y.F. is supported by an ETH Research Grant (grant no. 4412-1); I.P. is supported by an EMBO long-term fellowship (EMBO ALTF2014); and A.K. acknowledges SystemsX.ch for funding.
Author information
Authors and Affiliations
Contributions
S.S., A.K. and P.P. wrote the paper. Y.F. developed and optimized the original version of the protocol. S.S., P.L., O.M. and I.P. contributed to protocol optimization. I.P. and P.L. generated figures. P.J.B. contributed to editing of the manuscript and setup of the MS pipeline. A.K. developed and optimized algorithms and tools for protein structural analyses and prepared the user guide. P.P. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Effect of different chaotropes.
(A-D) Protein extracts from S. cerevisiae cells were subjected to the LiP-MS protocol with PK. LiP was conducted for one minute at a E/S of 1/100. After the LiP step, sodium deoxycholate (DOC) or urea were added to denature protein fragments (see conditions in Box 1) and complete digestion was obtained by the sequential addition of Lys-C and trypsin. The resulting peptides from LiP-treated and control samples were analyzed by LC-MS/MS. (A) The total number of proteins and (B) peptides, (C) the percent of missed-cleavage (MC) and (D) of half-tryptic (HT) peptides out of the total number of peptides identified are reported for the different treatments.
Supplementary Figure 2 Effect of protease incubation times in the LiP step.
(A-D) Protein extracts from S. cerevisiae cells were subjected to the LiP-MS protocol with PK. LiP was conducted at an E/S of 1/100, using different incubation times as indicated. After the LiP step, sodium deoxycholate (DOC) was added to denature protein fragments as indicated in the protocol and complete digestion was obtained by the sequential addition of Lys-C and trypsin. The resulting peptides from LiP-treated and control samples were analyzed by LC-MS/MS. (A) The total number of proteins and (B) peptides, (C) the percent of missed-cleavage (MC) and (D) of half-tryptic (HT) peptides out of the total number of peptides identified are reported for the different treatments.
Supplementary Figure 3 Devices to stabilize sample tubes in a boiling water bath.
Crucial to the proper quenching of PK is obtaining consistent and maximal heat throughout the water bath. We recommend using a metallic water bath for optimal heat transfer. To hold sample tubes inside the water bath, we recommend using a homebuilt device such as the one shown in (A). Alternatively, floating racks such as the ones shown in B) can be used. Depending on their material, floating racks such as those shown in (B) may deteriorate with heat. We recommend measuring the temperature of the boiling water bath and ensuring that it is above 95°C to achieve complete inactivation of PK after the first proteolysis step. Water levels should be sufficient to submerge sample tubes above the sample level, but floating of tubes should be avoided. Note that the water level in the water bath will decrease over prolonged use with serial quenching of multiple samples or sample batches.
Supplementary Figure 4 Examples of data visualization at the protein and pathway level.
(A) Three-dimensional structure of horse myoglobin (Protein Data Bank entry 2frf) shown in a grey cartoon representation, which highlights its mainly alpha-helical secondary structure. Protein regions for which LiP peptides were identified after LiP of a sample containing apomyoglobin are displayed in yellow, while half-tryptic peptide ends are coloured in red. The bound heme molecule is represented as a multicolored ball and stick model. Note the two half-tryptic peptide ends embedded in the helix F next to the heme molecule. The two half-tryptic peptides are increasing in abundance or become detectable in samples containing apomyoglobin, relative to samples containing holomyoglobin, since in apomyoglobin the F helix is replaced by a locally unfolded segment, easily cleaved by PK (see also Feng et al., 2014). (B) LiP-MS analysis of core carbon metabolism upon a shift from glycolytic to gluconeogenic growth, adapted from Feng et al., 2014. A schematic representation of core carbon metabolism in S. cerevisiae, comprising the glycolytic pathway, the TCA and glyoxylate cycles, and the ethanol production branch is shown. Proteins are colored according to the results of LiP-MS and protein abundance measurements by LC-MS. Abundance changes are differences between levels in gluconeogenic relative to glycolytic growth. Only significant abundance changes are reported. Protein abundance changes were determined based on measurements conducted on the trypsin control sample. Changes in the LiP pattern were calculated from data obtained on the LiP-treated sample and after normalization for protein abundance changes. Reproduced with permission from Feng et al., Global analysis of protein structural changes in complex proteomes. Nat. Biotechnol. 32, 1036–1044 (2014), Nature Publishing Group.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4. (PDF 597 kb)
Rights and permissions
About this article
Cite this article
Schopper, S., Kahraman, A., Leuenberger, P. et al. Measuring protein structural changes on a proteome-wide scale using limited proteolysis-coupled mass spectrometry. Nat Protoc 12, 2391–2410 (2017). https://doi.org/10.1038/nprot.2017.100
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.100
This article is cited by
-
In situ analysis of osmolyte mechanisms of proteome thermal stabilization
Nature Chemical Biology (2024)
-
Systematic identification of 20S proteasome substrates
Molecular Systems Biology (2024)
-
In planta expression of human polyQ-expanded huntingtin fragment reveals mechanisms to prevent disease-related protein aggregation
Nature Aging (2023)
-
Proteome-wide structural changes measured with limited proteolysis-mass spectrometry: an advanced protocol for high-throughput applications
Nature Protocols (2023)
-
Outer membrane vesicles from a mosquito commensal mediate targeted killing of Plasmodium parasites via the phosphatidylcholine scavenging pathway
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.