Abstract
Drosophila is widely used for the dissection of genetic and neuronal mechanisms of behavior. Recently, flies have emerged as a model for investigating the regulation of feeding and sleep. Although typically studied in isolation, increasing evidence points to a fundamental connection between these behaviors. Thus, a system for measuring sleep and feeding simultaneously in a single integrated system is important for interpreting behavioral shifts of either state. Here, we describe the construction and use of the Activity Recording Capillary Feeder or CAFE (ARC), a machine-vision (automated image tracking)-based system for the integrated measurement of sleep and feeding in individual Drosophila. Flies feed on liquid food from a microcapillary, and consumption is measured by tracking the liquid meniscus over time. Sleep measurements are obtained from positional tracking of individual animals, and arousal threshold can be determined by vibrational stimulus response. Using this system, a single computer and experimenter can track diverse behaviors from up to 60 individual flies in a single integrated system. The ARC is efficiently assembled with minimal training, and each experiment can be run for up to ∼7 d, with a total setup and breakdown time of ∼2 h.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
MacFadyen, U.M., Oswald, I. & Lewis, S.A. Starvation and human slow-wave sleep. J. Appl. Physiol. 35, 391–394 (1973).
Dario, A.J., Lopes, P.R., Freitas, C.G., Paschoalini, M.A. & Marino-Neto, J. Electrographic patterns of postprandial sleep after food deprivation or intraventricular adrenaline injections in pigeons. Brain Res. Bull. 39, 249–254 (1996).
Keene, A.C. et al. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr. Biol. 20, 1209–1215 (2010).
Murphy, K.R. et al. Postprandial sleep mechanics in Drosophila. eLife 5, e19334 (2016).
Linford, N.J., Chan, T.P. & Pletcher, S.D. Re-patterning sleep architecture in Drosophila through gustatory perception and nutritional quality. PLoS Genet. 8, e1002668 (2012).
Catterson, J.H. et al. Dietary modulation of Drosophila sleep-wake behaviour. PLoS ONE 5, e12062 (2010).
Wells, A.S., Read, N.W., Uvnas-Moberg, K. & Alster, P. Influences of fat and carbohydrate on postprandial sleepiness, mood, and hormones. Physiol. Behav. 61, 679–686 (1997).
Landstrom, U., Knutsson, A., Lennernas, M. & Soderberg, L. Laboratory studies of the effects of carbohydrate consumption on wakefulness. Nutr. Health 13, 213–225 (2000).
Grandner, M.A., Kripke, D.F., Naidoo, N. & Langer, R.D. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 11, 180–184 (2010).
Koban, M., Sita, L.V., Le, W.W. & Hoffman, G.E. Sleep deprivation of rats: the hyperphagic response is real. Sleep 31, 927–933 (2008).
Spiegel, K., Tasali, E., Penev, P. & Van Cauter, E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 141, 846–850 (2004).
Cirelli, C. & Bushey, D. Sleep and wakefulness in Drosophila melanogaster. Ann. N. Y. Acad. Sci. 1129, 323–329 (2008).
Artiushin, G. & Sehgal, A. The Drosophila circuitry of sleep-wake regulation. Curr. Opin. Neurobiol. 44, 243–250 (2017).
Greenspan, R.J., Tononi, G., Cirelli, C. & Shaw, P.J. Sleep and the fruit fly. Trends Neurosci. 24, 142–145 (2001).
Shaw, P.J., Cirelli, C., Greenspan, R.J. & Tononi, G. Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837 (2000).
Hendricks, J.C. et al. Rest in Drosophila is a sleep-like state. Neuron 25, 129–138 (2000).
van Alphen, B., Yap, M.H., Kirszenblat, L., Kottler, B. & van Swinderen, B. A dynamic deep sleep stage in Drosophila. J. Neurosci. 33, 6917–6927 (2013).
Donlea, J.M., Thimgan, M.S., Suzuki, Y., Gottschalk, L. & Shaw, P.J. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science 332, 1571–1576 (2011).
Shaw, P.J., Tononi, G., Greenspan, R.J. & Robinson, D.F. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature 417, 287–291 (2002).
Berry, J.A., Cervantes-Sandoval, I., Chakraborty, M. & Davis, R.L. Sleep facilitates memory by blocking dopamine neuron-mediated forgetting. Cell 161, 1656–1667 (2015).
Deshpande, S.A. et al. Quantifying Drosophila food intake: comparative analysis of current methodology. Nat. Methods 11, 535–540 (2014).
Yapici, N., Cohn, R., Schusterreiter, C., Ruta, V. & Vosshall, L.B. A taste circuit that regulates ingestion by integrating food and hunger signals. Cell 165, 715–729 (2016).
Ro, J., Harvanek, Z.M. & Pletcher, S.D. FLIC: high-throughput, continuous analysis of feeding behaviors in Drosophila. PLoS ONE 9, e101107 (2014).
Itskov, P.M. et al. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat. Commun. 5, 4560 (2014).
Bradski, G. The OpenCV library. Doctor Dobbs J. 25, 120–126 (2000).
Kabra, M., Robie, A.A., Rivera-Alba, M., Branson, S. & Branson, K. JAABA: interactive machine learning for automatic annotation of animal behavior. Nat. Methods 10, 64–67 (2013).
Pfeiffenberger, C., Lear, B.C., Keegan, K.P. & Allada, R. Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb. Protoc. http://dx.doi.org/10.1101/pdb.prot5518 (2010).
Ja, W.W. et al. Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proc. Natl. Acad. Sci. USA 106, 18633–18637 (2009).
Gilestro, G.F. Video tracking and analysis of sleep in Drosophila melanogaster. Nat. Protoc. 7, 995–1007 (2012).
Ja, W.W. et al. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. USA 104, 8253–8256 (2007).
Faville, R., Kottler, B., Goodhill, G.J., Shaw, P.J. & van Swinderen, B. How deeply does your mutant sleep? Probing arousal to better understand sleep defects in Drosophila. Sci. Rep. 5, 8454 (2015).
Donelson, N.C. et al. High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the 'tracker' program. PLoS ONE 7, e37250 (2012).
Xu, K., Zheng, X. & Sehgal, A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 8, 289–300 (2008).
Acknowledgements
We thank J. Jacobs and A. Sehgal for comments on the manuscript. This work was funded by the National Institutes of Health (R21DK092735 to W.W.J.).
Author information
Authors and Affiliations
Contributions
K.R.M., J.H.P., R.H., and W.W.J. contributed to the conception, development, and testing of the ARC; K.R.M., R.H., and W.W.J. wrote the manuscript; K.R.M., J.H.P., R.H., and W.W.J. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
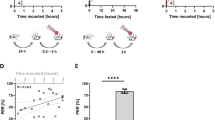
Supplementary Figure 1 Comparison of data between Expresso and ARC.
(a) Total ingestion volume and calorie intake for 33 minutes on the indicated diet following 24 hours of starvation, measured in the Expresso. n = 20 male flies. Data and figure were adapted from Yapici et al. 2016. (b) Replication of Expresso experiment using the ARC. n = 15 Canton-S males. Box and whiskers represent mean with 95% confidence intervals; blue circles represent mean ± s.e.m. a adapted with permission from ref. 22, Elsevier.
Supplementary Figure 2 ARC data output structure.
Mock data portraying 7 reads from the ARC. Each row represents a single read and each column is organized by the repeating structure shown. Labels (red) do not appear in the output file, whereas data (black) will appear in a tab-delimited format. The final column (Stimulus Delivered) will only appear in the data file if the optional arousal threshold module is run.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2. (PDF 332 kb)
Supplementary Data
Compressed archive of ARC software. Archive contains seven .stl files for 3D printing of ARC components, jArduino firmware, the JavaGrinders framework and the ARC modules (ARCController and ARCControllerMultiCam), the Noah analysis program (Python-based), and a sample ARC data set of 30 Canton-S males over 2 d. (ZIP 28593 kb)
Video guide to using the ARC.
Overview of the procedure. (MP4 31023 kb)
Rights and permissions
About this article
Cite this article
Murphy, K., Park, J., Huber, R. et al. Simultaneous measurement of sleep and feeding in individual Drosophila. Nat Protoc 12, 2355–2359 (2017). https://doi.org/10.1038/nprot.2017.096
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.096
This article is cited by
-
Kalium channelrhodopsins effectively inhibit neurons
Nature Communications (2024)
-
Circadian autophagy drives iTRF-mediated longevity
Nature (2021)
-
Chronic social isolation signals starvation and reduces sleep in Drosophila
Nature (2021)
-
Expansion and application of dye tracers for measuring solid food intake and food preference in Drosophila
Scientific Reports (2021)
-
Neurofibromin regulates metabolic rate via neuronal mechanisms in Drosophila
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.