Abstract
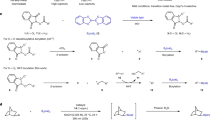
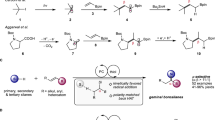
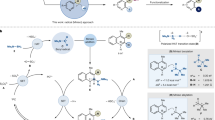
Boronic acids and esters have critical roles in the areas of synthetic organic chemistry, molecular sensors, materials science, drug discovery, and catalysis. Many of the current applications of boronic acids and esters require materials with very low levels of transition metal contamination. Most of the current methods for the synthesis of boronic acids, however, require transition metal catalysts and ligands that must be removed via additional purification procedures. This protocol describes a simple, metal- and additive-free method of conversion of haloarenes directly to boronic acids and esters. This photoinduced borylation protocol does not require expensive and toxic metal catalysts or ligands, and it produces innocuous and easy-to-remove by-products. Furthermore, the reaction can be carried out on multigram scales in common-grade solvents without the need for reaction mixtures to be deoxygenated. The setup and purification steps are typically accomplished within 1–3 h. The reactions can be run overnight, and the protocol can be completed within 13–16 h. Two representative procedures that are described in this protocol provide details for preparation of a boronic acid (3-cyanopheylboronic acid) and a boronic ester (1,4-benzenediboronic acid bis(pinacol)ester). We also discuss additional details of the method that will be helpful in the application of the protocol to other haloarene substrates.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Suzuki, A. & Brown, H.C. Organic Syntheses via Boranes Vol. 3 (Aldrich Chemical Company, 2003).
Boronic Acids 2nd edn. (ed. Hall, D.G.) (Wiley-VCH, 2011).
Gutekunst, W.R. & Baran, P.S. C–H functionalization logic in total synthesis. Chem. Soc. Rev. 40, 1976–1991 (2011).
Corey, E.J. Catalytic enantioselective Diels-Alder reactions: methods, mechanistic fundamentals, pathways, and applications. Angew. Chem. Int. Ed. 41, 1650–1667 (2002).
Dimitrijević, E. & Taylor, M.S. Organoboron acids and their derivatives as catalysts for organic synthesis. ACS Catal. 3, 945–962 (2013).
Ishihara, K. Synthesis and application of organoboron compounds. Top. Organomet. Chem. 49, 243–270 (2015).
Lorbach, A., Huebner, A. & Wagner, M. Aryl(hydro)boranes: versatile building blocks for boron-doped π-electron materials. Dalton Trans. 41, 6048–6063 (2012).
Jäkle, F. Recent advances in the synthesis and applications of organoborane polymers. Top. Organomet. Chem. 49, 297–325 (2015).
Trippier, P.C. & McGuigan, C. Boronic acids in medicinal chemistry: anticancer, antibacterial and antiviral applications. Med. Chem. Commun. 1, 183–198 (2010).
Yang, W., Gao, W. & Wang, B. Applications of boronic acids in chemical biology and medicinal chemistry. In Boronic Acids 2nd edn. (ed. Hall, D.G.) 591–619 (Wiley-VCH, 2011).
Ban, H.S. & Nakamura, H. Boron-based drug design. Chem. Rec. 15, 616–635 (2015).
Fujita, N., Shinkai, S. & James, T.D. Boronic acids in molecular self-assembly. Chem. Asian J. 3, 1076–1091 (2008).
Mastalerz, M. Shape-persistent organic cage compounds by dynamic covalent bond formation. Angew. Chem. Int. Ed. 49, 5042–5053 (2010).
James, T.D. & Shinkai, S. Artificial receptors as chemosensors for carbohydrates. Top. Curr. Chem. 218, 159–200 (2002).
Jelinek, R. & Kolusheva, S. Carbohydrate biosensors. Chem. Rev. 104, 5987–6015 (2004).
Pal, A., Berube, M. & Hall, D.G. Design, synthesis, and screening of a library of peptidyl bis-boroxoles as low molecular weight receptors for complex oligosaccharides in water: identification of a receptor for the tumour marker TF-antigen. Angew. Chem. Int. Ed. 49, 1492–1495 (2010).
Wade, C.R., Broomsgrove, A.E.J., Aldridge, S. & Gabbaï, F.P. Fluoride ion complexation and sensing using organoboron compounds. Chem. Rev. 110, 3958–3984 (2010).
Wu, X. et al. Selective sensing of saccharides using simple boronic acids and their aggregates. Chem. Soc. Rev. 42, 8032–8048 (2013).
Guan, Y. & Zhang, Y. Boronic acid-containing hydrogels: synthesis and their applications. Chem. Soc. Rev. 42, 8106–8121 (2013).
You, L., Zha, D. & Anslyn, E.V. Recent advances in supramolecular analytical chemistry using optical sensing. Chem. Rev. 115, 7840–7892 (2015).
Bunnett, J.F. Aromatic substitution by the SRN1 mechanism. Acc. Chem. Res. 11, 413–420 (1978).
Uyeda, C., Tan, Y.C., Fu, G.C. & Peters, J.C. A new family of nucleophiles for photoinduced, copper-catalyzed cross-couplings via single-electron transfer: reactions of thiols with aryl halides under mild conditions (0 °C). J. Am. Chem. Soc. 135, 9548–9552 (2013).
Li, L. et al. Photoinduced metal-catalyst-free aromatic Finkelstein reaction. J. Am. Chem. Soc. 137, 8328–8331 (2015).
Chen, K., He, P., Zhang, S. & Li, P. Synthesis of aryl trimethylstannanes from aryl halides: an efficient photochemical method. Chem. Commun. 52, 9125–9128 (2016).
Mella, M. et al. Photoinduced, ionic Meerwein arylation of olefins. J. Org. Chem. 66, 6344–6352 (2001).
Dichiarante, V., Fagnoni, M. & Albini, A. Metal-free synthesis of sterically crowded biphenyls by direct Ar–H substitution in alkyl benzenes. Angew. Chem. Int. Ed. 46, 6495–6498 (2007).
Grimshaw, J. & de Silva, A.P. Photochemistry and photocyclization of aryl halides. Chem. Soc. Rev. 10, 181–203 (1981).
Lu, S.C. et al. Intramolecular photochemical cross-coupling reactions of 3-acyl-2-haloindoles and 2-chloropyrrole-3-carbaldehydes with substituted benzenes. Adv. Synth. Catal. 351, 2839–2844 (2009).
Mfuh, A.M., Doyle, J.D., Chhetri, B., Arman, H.D. & Larionov, O.V. Scalable, metal- and additive-free, photoinduced borylation of haloarenes and quaternary arylammonium salts. J. Am. Chem. Soc. 138, 2985–2988 (2016).
Chen, K., Zhang, S., He, P. & Li, P. Efficient metal-free photochemical borylation of aryl halides under batch and continuous-flow conditions. Chem. Sci. 7, 3676–3680 (2016).
Chen, K., Cheung, M.S., Lin, Z. & Li, P. Metal-free borylation of electron-rich aryl (pseudo)halides under continuous-flow photolytic conditions. Org. Chem. Front. 3, 875–879 (2016).
Mfuh, A.M. et al. Additive- and metal-free, predictably 1,2- and 1,3-regioselective, photoinduced dual C–H/C–X borylation of haloarenes. J. Am. Chem. Soc. 138, 8408–8411 (2016).
Li, G. et al. Elemental impurities in pharmaceutical excipients. J. Pharm. Sci. 104, 4197–4206 (2015).
McDaniel, F.D., Datar, S.A., Nigam, M. & Ravi Prasad, G.V. Impurity measurements in semiconductor materials using trace element accelerator mass spectrometry. Nucl. Instrum. Methods Phys. Res., Sect. B 190, 826–830 (2002).
Ishiyama, T., Murata, M. & Miyaura, N. Palladium(0)-catalyzed cross-coupling reaction of alkoxydiboron with haloarenes: a direct procedure for arylboronic esters. J. Org. Chem. 60, 7508–7510 (1995).
Murata, M., Watanabe, S. & Masuda, Y. Novel palladium(0)-catalyzed coupling reaction of dialkoxyborane with aryl halides: convenient synthetic route to arylboronates. J. Org. Chem. 62, 6458–6459 (1997).
Ishiyama, T. & Miyaura, N. Chemistry of Group 13 element-transition metal linkage — the platinum- and palladium-catalyzed reactions of (alkoxo)diborons. J. Organomet. Chem. 611, 392–402 (2000).
Chow, W.K. et al. A decade advancement of transition metal-catalyzed borylation of aryl halides and sulfonates. RSC Adv. 3, 12518–12539 (2013).
Cho, J.-Y., Tse, M.K., Holmes, D., Maleczka, R.E. & Smith, M.R. Remarkably selective iridium catalysts for the elaboration of aromatic C–H bonds. Science 295, 305–308 (2002).
Mkhalid, I.A.I., Barnard, J.H., Marder, T.B., Murphy, J.M. & Hartwig, J.F. CH activation for the construction of CB bonds. Chem. Rev. 110, 890–931 (2010).
Hartwig, J.F. Borylation and silylation of C–H bonds: a platform for diverse C–H bond functionalizations. Acc. Chem. Res. 45, 864–873 (2012).
Acknowledgements
O.V.L. gratefully acknowledges financial support from the Welch Foundation (AX-1788), the National Science Foundation (NSF; CHE-1455061), the National Institute of General Medical Sciences (NIGMS; SC3GM105579), and the University of Texas at San Antonio.
Author information
Authors and Affiliations
Contributions
A.M.M., B.D.S., and W.C. carried out the experiments. O.V.L. and A.M.M. designed the experiments and analyzed the data. O.V.L. directed the research. O.V.L. and A.M.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary information
Supplementary Figures
Supplementary Figures 1–4 (PDF 275 kb)
Rights and permissions
About this article
Cite this article
Mfuh, A., Schneider, B., Cruces, W. et al. Metal- and additive-free photoinduced borylation of haloarenes. Nat Protoc 12, 604–610 (2017). https://doi.org/10.1038/nprot.2016.184
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.184
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.