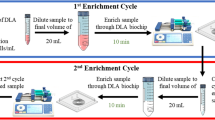
Abstract
Enumerating specific cell types from whole blood can be very useful for research and diagnostic purposes—e.g., for counting of CD4 and CD8 T cells in HIV/AIDS diagnostics. We have developed a biosensor based on a differential immunocapture technology to enumerate specific cells in 30 min using 10 μl of blood. This paper provides a comprehensive stepwise protocol to replicate our biosensor for CD4 and CD8 cell counts. The biochip can also be adapted to enumerate other specific cell types such as somatic cells or cells from tissue or liquid biopsies. Capture of other specific cells requires immobilization of their corresponding antibodies within the capture chamber. Therefore, this protocol is useful for research into areas surrounding immunocapture-based biosensor development. The biosensor production requires 24 h, a one-time cell capture optimization takes 6–9 h, and the final cell counting experiment in a laboratory environment requires 30 min to complete.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Global HIV/AIDS Response. Epidemic update and health sector progress towards Universal Access. World Health Organization (2011).
Damhorst, G., Watkins, N.N. & Bashir, R. Micro and nanotechnology for HIV/AIDS diagnostics in resource-limited settings. IEEE Trans. Biomed. Eng. 60, 715–726 (2013).
Damhorst, G., Murtagh, M., Rodriguez, W.R. & Bashir, R. Microfluidics and nanotechnology for detection of global infectious diseases. Proc. IEEE 103, 150–160 (2015).
Rowley, C.F. Developments in CD4 and viral load monitoring in resource-limited settings. Clin. Infect. Dis. 58, 407–412 (2014).
Pahwa, S. et al. CD4+/CD8+ T cell ratio for diagnosis of HIV-1 infection in infants: women and infants transmission study. Pediatrics 122, 331–339 (2008).
Taylor, J.M., Fahey, J.L., Detels, R. & Giorgi, J.V. CD4 percentage, CD4 number, and CD4:CD8 ratio in HIV infection: which to choose and how to choose. J. AIDS 2, 114–124 (1989).
Wang, J.H., Wang, C.H., Lin, C.C., Lei, H.Y. & Lee, G.B. An integrated microfluidic system for counting of CD4+/CD8+ T lymphocytes. Microfluid. Nanofluid. 10, 531–541 (2011).
Gohring, J.T. & Fan, X. Label free detection of CD4+ and CD8+ T lymphocytes with the optofluidic ring resonator biosensor. Sensors 10, 5798–5808 (2010).
Carey, J.L., McCoy, J.P. Jr. & Keren, D.F. Flow Cytometry in Clinical Diagnosis 4th edn. American Society for Clinical Pathology, 2007.
Srithanaviboonchai, K. et al. Novel low-cost assay for the monitoring of CD4 counts in HIV-infected individuals. J. Acquir. Immune Defic. Syndr. 47, 135–139 (2008).
Crowe, S., Turnbull, S., Oelrichs, R. & Dunne, A. Monitoring of human immunodeficiency virus infection in resource-constrained countries. Clin. Infect. Dis. 37, S25–S35 (2003).
Coulter, W.H. Means for counting particles suspended in a fluid. US Patent 2656508 (1953).
Koch, M., Evans, A. & Brunnschweiler, A. Design and fabrication of a micromachined Coulter counter. J. Micromech. Microeng. 9, 159–161 (1999).
Holmes, D. et al. Leukocyte analysis and differentiation using high speed microfluidic single cell impedance cytometry. Lab Chip 9, 2881–2889 (2009).
Hassan, U. Microfluidic sensor for white blood cell counting and flow metering. M.S. thesis, Electrical & Computer Engineering, University of Illinois at Urbana-Champaign (2013).
Ellappan, P. & Sundararaian, R. A simulation study of a electrical model of a biological cell. J. Electrostat. 63, 297–309 (2005).
Hughes, M.P. AC electrokinetics: applications for nanotechnology. Nanotechnology 11, 124–132 (2000).
Gawad, S., Schild, L. & Renaud, P. Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab Chip 1, 76–82 (2001).
Pierzchalski, A., Hebeisen, M., Mittag, A., Berardino, M.D. & Tarnok, A. Label-free single cell analysis with a chip-based impedance flow cytometer. Proc. SPIE 75681B doi:10.1117/12.840865 (2010).
Cheung, K.C. et al. Microfluidic impedance-based flow cytometry. Cytometry A 77, 648–666 (2010).
Cheng, X. et al. Enhancing the performance of a point-of-care CD4+ T cell counting microchip through monocyte depletion for HIV/AIDS diagnostics. Lab Chip 9, 1357–1364 (2009).
Beck, M. et al. On-chip sample preparation by controlled release of antibodies for simple CD4 counting. Lab Chip 12, 167–173 (2012).
Smith, Z.J. et al. Single-step preparation and image based counting of minute volumes of human blood. Lab Chip 14, 3029 (2014).
Boyle, D.S., Hawkins, K.R., Steele, M.S., Singhal, M. & Cheng, X. Emerging technologies for point-of-care CD4 T-lymphocyte counting. Trends Biotechnol. 30, 1 (2012).
Schade-Kampmann, G., Huwiler, A., Hebeisen, M., Hessler, T. & Di Berardino, M. On-chip non-invasive and label-free cell discrimination by impedance spectroscopy. Cell Prolif. 41, 830–840 (2008).
Cheng, X. et al. Cell detection and counting through cell lysate impedance spectroscopy in microfluidic devices. Lab Chip 7, 746–755 (2007).
Watkins, N.N., Venkatesan, B.M., Toner, M., Rodriguez, W. & Bashir, R. A robust electrical microcytometer with 3-dimensional hydrofocusing. Lab Chip 9, 3177–3184 (2009).
Watkins, N.N. et al. microfabricated electrical differential counter for the selective enumeration of CD4+ T lymphocytes. Lab Chip 11, 437–447 (2011).
Watkins, N.N. et al. Microfluidic CD4+ and CD8+ T lymphocyte counters for point-of-care HIV diagnostics using whole blood. Sci. Transl. Med. 5, 214ra170 (2013).
Hassan, U., Watkins, N., Edwards, C. & Bashir, R. Flow metering characterization within an electrical cell counting microfluidic device. Lab Chip 14, 1469 (2014).
Hassan, U. & Bashir, R. Coincidence detection of heterogeneous cell populations from whole blood with coplanar electrodes in a microfluidic impedance cytometer. Lab Chip 14, 4370–4381 (2014).
Hassan, U. & Bashir, R. Electrical cell counting process characterization in a microfluidic impedance cytometer. Biomed. Microdevices 16, 697–704 (2014).
Acknowledgements
The authors thank A. Vaid at Champaign-Urbana Public Health District (CUPHD) for providing the HIV-infected blood samples; and C. Edwards, L. Orlandic and C. Yang for PDMS device fabrication. The authors acknowledge the support of Center for Integration of Medicine and Innovative Technology (CIMIT)'s Point-of-Care Technology Center in Primary Care (POCTRN) Grant and funding from University of Illinois at Urbana-Champaign.
Author information
Authors and Affiliations
Contributions
U.H., N.N.W., B.R. and R.B. designed the study. U.H., N.N.W. and G.D. performed the experiments. U.H. wrote the paper and R.B. edited and proofread the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Differential counting Biochip
Image of the integrated biochip for specific leukocyte counting by differential immuno-capture technique. The footprint of the chip is 3 cm x 4 cm.
Supplementary Figure 2 Schematic of the electrical counter module.
(a) Schematic shows both entrance and exit counters. Holes can be punched in circular spots to be inlets and outlets of counters. The spacing and width of the counter pads are 2500 μm and 1500 μm respectively. (b) Shows the zoomed-in dotted region in (a). The top view of the counter with dimensions of the electrodes is shown. The width of the electrodes and the spacing in between the electrodes is 15 μm. (c) The cross-sectional view of the counter. The counting height is 15 μm, however rest of the fluidic channel height is 115 μm.
Supplementary Figure 3 Electrical cell pulse.
The counting channel cross-sectional view showing the cell flowing over the electrodes. The corresponding voltage pulse generated as a result of a cell transitioning through the counting channel over the electrodes. Adapted from Hassan, U. & Bashir, R. Coincidence detection of heterogeneous cell populations from whole blood with coplanar electrodes in a microfluidic impedance cytometer. Lab Chip 14, 4370–4381 (2014).
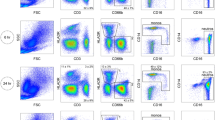
Supplementary Figure 6 Flow cytometry analysis of the labeled cells.
(a) The Side scatter (SSC) vs. CD45 plot shows a distinct population of lymphocytes, which can be gated out in the plot. (b) The gated lymphocyte population in (a) is plotted in a CD3 vs. CD4 fluorescent plot. The top right quadrant gives the CD4 lymphocytes. Similarly, bottom right gives CD4 monocytes.
Supplementary Figure 7 Schematic of individual modules of the biochip.
1. The cell-lysing module. Quenching, lysing and blood will be infused at a, b and c inlets ports respectively as shown. 2. The electrical counters module. 3. Capture chamber module. The inlet and outlet ports of each module are shown too.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 1297 kb)
Supplementary Data
AutoCAD design file (BiochipDesign_NP.dwg); Lock-in amplifier settings file (lockin.zicfg); LabVIEW file for Data Acquisition (Counter_Differential_NP.vi) (ZIP 18372 kb)
Rights and permissions
About this article
Cite this article
Hassan, U., Watkins, N., Reddy, B. et al. Microfluidic differential immunocapture biochip for specific leukocyte counting. Nat Protoc 11, 714–726 (2016). https://doi.org/10.1038/nprot.2016.038
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.038
This article is cited by
-
Thin flexible lab-on-a-film for impedimetric sensing in biomedical applications
Scientific Reports (2022)
-
Biochip with multi-planar electrodes geometry for differentiation of non-spherical bioparticles in a microchannel
Scientific Reports (2021)
-
Simultaneous electrical detection of IL-6 and PCT using a microfluidic biochip platform
Biomedical Microdevices (2020)
-
Design and analysis of microfluidic cell counter using spice simulation
SN Applied Sciences (2019)
-
Point-of-care sensors for the management of sepsis
Nature Biomedical Engineering (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.