Abstract
Microbial infections are a global health problem, particularly as microbes are continually developing resistance to antimicrobial treatments. An effective and reliable method for testing the virulence of different microbial pathogens is therefore a useful research tool. This protocol describes how the chicken embryo can be used as a trustworthy, inexpensive, ethically desirable and quickly accessible model to assess the virulence of the human bacterial pathogen Listeria monocytogenes, which can also be extended to other microbial pathogens. We provide a step-by-step protocol and figures and videos detailing the method, including egg handling, infection strategies, pathogenicity screening and isolation of infected organs. From the start of incubation of the fertilized eggs, the protocol takes <4 weeks to complete, with the infection part taking only 3 d. We discuss the appropriate controls to use and potential adjustments needed for adapting the protocol for other microbial pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hamon, M., Bierne, H. & Cossart, P. Listeria monocytogenes: a multifaceted model. Nat. Rev. Microbiol. 4, 423–434 (2006).
Drevets, D.A. & Bronze, M.S. Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol. Med. Microbiol. 53, 151–165 (2008).
Vazquez-Boland, J.A. et al. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14, 584–640 (2001).
Cossart, P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 108, 19484–19491 (2011).
Schnupf, P. & Portnoy, D.A. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 9, 1176–1187 (2007).
Freitag, N.E., Port, G.C. & Miner, M.D. Listeria monocytogenes—from saprophyte to intracellular pathogen. Nat. Rev. Microbiol. 7, 623–628 (2009).
Scortti, M., Monzo, H.J., Lacharme-Lora, L., Lewis, D.A. & Vazquez-Boland, J.A. The PrfA virulence regulon. Microbes Infect. 9, 1196–1207 (2007).
Chakraborty, T. et al. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J. Bacteriol. 174, 568–574 (1992).
Freitag, N.E., Rong, L. & Portnoy, D.A. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect. Immun. 61, 2537–2544 (1993).
Gripenland, J., Andersson, C. & Johansson, J. Exploring the chicken embryo as a possible model for studying Listeria monocytogenes pathogenicity. Front. Cell. Infect. Microbiol. 4, 170 (2014).
Disson, O. & Lecuit, M. In vitro and in vivo models to study human listeriosis: mind the gap. Microbes Infect. 15, 971–980 (2013).
Disson, O. et al. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature 455, 1114–1118 (2008).
Lecuit, M. et al. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292, 1722–1725 (2001).
Smith, M.A. et al. Dose-response model for Listeria monocytogenes-induced stillbirths in nonhuman primates. Infect. Immun. 76, 726–731 (2008).
D'Orazio, S.E. Animal models for oral transmission of Listeria monocytogenes. Front. Cell. Infect. Microbiol. 4, 15 (2014).
Mukherjee, K. et al. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl. Environ. Microbiol. 76, 310–317 (2010).
Mansfield, B.E., Dionne, M.S., Schneider, D.S. & Freitag, N.E. Exploration of host-pathogen interactions using Listeria monocytogenes and Drosophila melanogaster. Cell. Microbiol. 5, 901–911 (2003).
Levraud, J.P. et al. Real-time observation of Listeria monocytogenes-phagocyte interactions in living zebrafish larvae. Infect. Immun. 77, 3651–3660 (2009).
Thomsen, L.E., Slutz, S.S., Tan, M.W. & Ingmer, H. Caenorhabditis elegans is a model host for Listeria monocytogenes. Appl. Environ. Microbiol. 72, 1700–1701 (2006).
Jiang, L.L. et al. Characterization of a mutant Listeria monocytogenes strain expressing green fluorescent protein. Acta Biochim. Biophys. Sin. 37, 19–24 (2005).
Severino, P. et al. Comparative transcriptome analysis of Listeria monocytogenes strains of the two major lineages reveals differences in virulence, cell wall, and stress response. Appl. Environ. Microbiol. 73, 6078–6088 (2007).
Janse, E.M. & Jeurissen, S.H. Ontogeny and function of two non-lymphoid cell populations in the chicken embryo. Immunobiology 182, 472–481 (1991).
Nix, E.B. et al. Virulence of Francisella spp. in chicken embryos. Infect. Immun. 74, 4809–4816 (2006).
Polakowska, K. et al. The virulence of Staphylococcus aureus correlates with strain genotype in a chicken embryo model but not a nematode model. Microbes Infect. 14, 1352–1362 (2012).
Oh, J.Y. et al. The embryo lethality of Escherichia coli isolates and its relationship to the presence of virulence-associated genes. Poult. Sci. 91, 370–375 (2012).
Alnassan, A.A. et al. Embryonated chicken eggs as an alternative model for mixed Clostridium perfringens and Eimeria tenella infection in chickens. Parasitol. Res. 112, 2299–2306 (2013).
Jacobsen, I.D., Grosse, K., Berndt, A. & Hube, B. Pathogenesis of Candida albicans infections in the alternative chorio-allantoic membrane chicken embryo model resembles systemic murine infections. PloS One 6, e19741 (2011).
Jacobsen, I.D. et al. Embryonated eggs as an alternative infection model to investigate Aspergillus fumigatus virulence. Infect. Immun. 78, 2995–3006 (2010).
Osorio, J.E. et al. Characterization of West Nile viruses isolated from captive American flamingoes (Phoenicopterus ruber) in Medellin, Colombia. Am. J. Tropical Med. Hyg. 87, 565–572 (2012).
Takamatsu, Y. et al. NS1 protein expression facilitates production of Japanese encephalitis virus in avian cells and embryonated chicken eggs. J. Gen. Virol. 95, 373–383 (2014).
Glaser, P. et al. Comparative genomics of Listeria species. Science 294, 849–852 (2001).
Acknowledgements
J.J. was supported by Umeå University, the Swedish Research Council grant nos. K2011-56X-15144-08-6 and 621-2012-2451, the Knut and Alice Wallenberg Foundations, and European Research Council (ERC) starting grant no. 260764-RNAntibiotics.
Author information
Authors and Affiliations
Contributions
C.A. performed the experiments, analyzed the results and wrote the paper. J.G. established the first protocol and analyzed the results. J.J. analyzed the results and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
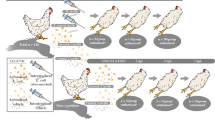
Extraction of liver from chicken embryo.
After the chicken embryo has been isolated from the egg (see Figure 6a-c), the head of the chicken embryo is removed before embryo is placed on its back, in order to facilitate the liver extraction. Carefully open up the abdomen using a pair of forceps and extract the liver (see also Figure 6d). This step could prove difficult and might require some training on non-infected embryos. All experiments were performed in compliance with Swedish regulations. (AVI 26120 kb)
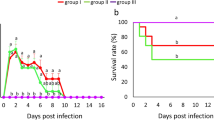
Monitoring chicken embryo survival.
By candling, the survival of a chicken embryo can be followed (see Figure 3b for setup). In this video, an alive chicken embryo is visualized (light torch on top). Please note the movement of the embryo and the well-defined capillaries (both thicker and thinner) inside the egg shell. Such capillaries are not observed in dead chicken embryos (compare Figure 3c and d). All experiments were performed in compliance with Swedish regulations. (AVI 23254 kb)
Infecting chicken embryos in their allantoic cavity.
Before perforation of the egg shell, the egg should be repeatedly disinfected using 70% ethanol. By a rapid movement, the egg shell is perforated using a pair of forceps (see also Figure 4a). The needle is inserted approximately 8 mm, through the chorioallantoic membrane at an angle of ∼45°C angle (see also Figure 4b). After the needle is removed, hot paraffin and tape is added onto the egg shell at the perforation site (see also Figure 4c and d). Remember to appropriately mark the egg for later identification. All experiments were performed in compliance with Swedish regulations. (AVI 25963 kb)
Rights and permissions
About this article
Cite this article
Andersson, C., Gripenland, J. & Johansson, J. Using the chicken embryo to assess virulence of Listeria monocytogenes and to model other microbial infections. Nat Protoc 10, 1155–1164 (2015). https://doi.org/10.1038/nprot.2015.073
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.073
This article is cited by
-
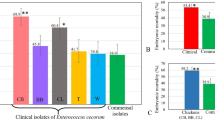
Chicken embryo lethality assay for determining the lethal dose, tissue distribution and pathogenicity of clinical Enterococcus cecorum isolates from poultry
Scientific Reports (2022)
-
Genetic and phenotypic analysis of the pathogenic potential of two novel Chlamydia gallinacea strains compared to Chlamydia psittaci
Scientific Reports (2021)
-
Role in virulence of phospholipases, listeriolysin O and listeriolysin S from epidemic Listeria monocytogenes using the chicken embryo infection model
Veterinary Research (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.