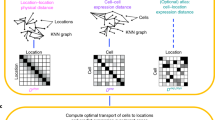
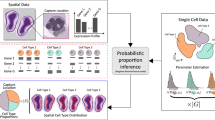
Abstract
Single-cell gene expression analysis has contributed to a better understanding of the transcriptional heterogeneity in a variety of model systems, including those used in research in developmental, cancer and stem cell biology. Nowadays, technological advances facilitate the generation of large gene expression data sets in high-throughput format. Strategies are needed to pertinently visualize this information in a tissue structure–related context, so as to improve data analysis and aid the drawing of meaningful conclusions. Here we describe an approach that uses spatial properties of the tissue source to enable the reconstruction of hollow sphere–shaped tissues and organs from single-cell gene expression data in 3D space. To demonstrate our method, we used cells of the mouse otocyst and the renal vesicle as examples. This protocol presents a straightforward computational expression analysis workflow, and it is implemented on the MATLAB and R statistical computing and graphics software platforms. Hands-on time for typical experiments can be <1 h using a standard desktop PC or Mac.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Guo, G. et al. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev. Cell 18, 675–685 (2010).
Dalerba, P. et al. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat. Biotechnol. 29, 1120–1127 (2011).
Moignard, V. et al. Characterization of transcriptional networks in blood stem and progenitor cells using high-throughput single-cell gene expression analysis. Nat. Cell Biol. 15, 363–372 (2013).
Guo, G. et al. Mapping cellular hierarchy by single-cell analysis of the cell surface repertoire. Cell Stem Cell 13, 492–505 (2013).
Narsinh, K.H. et al. Single-cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J. Clin. Invest. 121, 1217–1221 (2011).
Durruthy-Durruthy, R. et al. Reconstruction of the mouse otocyst and early neuroblast lineage at single-cell resolution. Cell 157, 964–978 (2014).
Brunskill, E.W. et al. Single-cell dissection of early kidney development: multilineage priming. Development 141, 3093–3101 (2014).
Elowitz, M.B., Levine, A.J., Siggia, E.D. & Swain, P.S. Stochastic gene expression in a single cell. Science 297, 1183–1186 (2002).
Yeung, K.Y. & Ruzzo, W.L. Principal component analysis for clustering gene expression data. Bioinformatics 17, 763–774 (2001).
Ringner, M. What is principal component analysis? Nat. Biotechnol. 26, 303–304 (2008).
Neves, S.R. et al. Cell shape and negative links in regulatory motifs together control spatial information flow in signaling networks. Cell 133, 666–680 (2008).
Fuhrmann, S. Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 93, 61–84 (2010).
Brewster, S.F. The development and differentiation of human seminal vesicles. J. Anat. 143, 45–55 (1985).
Essner, J.J., Amack, J.D., Nyholm, M.K., Harris, E.B. & Yost, H.J. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 132, 1247–1260 (2005).
Weninger, W.J. & Mohun, T. Phenotyping transgenic embryos: a rapid 3-D screening method based on episcopic fluorescence image capturing. Nat. Genet. 30, 59–65 (2002).
Schena, M., Shalon, D., Davis, R.W. & Brown, P.O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270, 467–470 (1995).
Fernandez, R. et al. Imaging plant growth in 4D: robust tissue reconstruction and lineaging at cell resolution. Nat. Methods 7, 547–553 (2010).
Lee, J.H. et al. Highly multiplexed subcellular RNA sequencing in situ. Science 343, 1360–1363 (2014).
Jolliffe, I.T. In Principal Component Analysis 2nd edn. (Springer, 2002).
Scholz, M., Kaplan, F., Guy, C.L., Kopka, J. & Selbig, J. Non-linear PCA: a missing data approach. Bioinformatics 21, 3887–3895 (2005).
Verpy, E., Leibovici, M. & Petit, C. Characterization of otoconin-95, the major protein of murine otoconia, provides insights into the formation of these inner ear biominerals. Proc. Natl. Acad. Sci. USA 96, 529–534 (1999).
Lin, Z., Cantos, R., Patente, M. & Wu, D.K. Gbx2 is required for the morphogenesis of the mouse inner ear: a downstream candidate of hindbrain signaling. Development 132, 2309–2318 (2005).
Hurd, E.A., Poucher, H.K., Cheng, K., Raphael, Y. & Martin, D.M. The ATP-dependent chromatin remodeling enzyme CHD7 regulates pro-neural gene expression and neurogenesis in the inner ear. Development 137, 3139–3150 (2010).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Citri, A., Pang, Z.P., Sudhof, T.C., Wernig, M. & Malenka, R.C. Comprehensive qPCR profiling of gene expression in single neuronal cells. Nat. Protoc. 7, 118–127 (2012).
Schmitz, B. et al. Magnetic activated cell sorting (MACS): a new immunomagnetic method for megakaryocytic cell isolation: comparison of different separation techniques. Eur. J. Haematol. 52, 267–275 (1994).
Espina, V. et al. Laser-capture microdissection. Nat. Protoc. 1, 586–603 (2006).
Tang, F. et al. mRNA-seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Pollen, A.A. et al. Low-coverage single-cell mRNA sequencing reveals cellular heterogeneity and activated signaling pathways in developing cerebral cortex. Nat. Biotechnol. 32, 1053–1058 (2014).
Sanchez-Freire, V., Ebert, A.D., Kalisky, T., Quake, S.R. & Wu, J.C. Microfluidic single-cell real-time PCR for comparative analysis of gene expression patterns. Nat. Protoc. 7, 829–838 (2012).
Acknowledgements
We thank all members of the Heller laboratory for comments on the manuscript. This work was supported by National Institutes of Health grants DC006167 and DC012250 to S.H., by P30 core support (DC010363), by the Stanford Initiative to Cure Hearing Loss and in part by FP7-Health-2013-Innovation, a cooperative grant by the European Commission.
Author information
Authors and Affiliations
Contributions
R.D.-D. and S.H. designed the experiments; R.D.-D. performed the experiments; R.D.-D. and A.G. performed data analysis; and R.D.-D., A.G. and S.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.H. is affiliated with Inception 3, Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Methods (PDF 818 kb)
Supplementary Software 1
Contains all function files for MATLAB. (ZIP 18 kb)
Supplementary Software 2
Contains script files for R (ZIP 13 kb)
Supplementary Data
Contains gene expression data (qRT-PCR for otocysts and RNA-seq for renal vesicles) in comma-separated values (CSV) format to be used in the protocol. (ZIP 2225 kb)
Rights and permissions
About this article
Cite this article
Durruthy-Durruthy, R., Gottlieb, A. & Heller, S. 3D computational reconstruction of tissues with hollow spherical morphologies using single-cell gene expression data. Nat Protoc 10, 459–474 (2015). https://doi.org/10.1038/nprot.2015.022
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.022
This article is cited by
-
Alpha cell receptor for advanced glycation end products associate with glucagon expression in type 1 diabetes
Scientific Reports (2023)
-
Human Cell Atlas and cell-type authentication for regenerative medicine
Experimental & Molecular Medicine (2020)
-
Novel computational model of gastrula morphogenesis to identify spatial discriminator genes by self-organizing map (SOM) clustering
Scientific Reports (2019)
-
Spatial transcriptomic analysis of cryosectioned tissue samples with Geo-seq
Nature Protocols (2017)
-
Revealing the vectors of cellular identity with single-cell genomics
Nature Biotechnology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.