Abstract
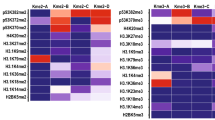
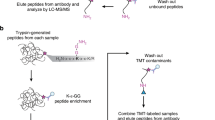
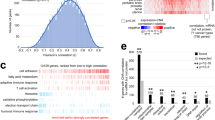
We present a protocol for using the triple malignant brain tumor domains of L3MBTL1 (3xMBT), which bind to mono- and di-methylated lysine with minimal sequence specificity, in order to enrich for such methylated lysine from cell lysates. Cells in culture are grown with amino acids containing light or heavy stable isotopic labels. Methylated proteins are enriched by incubating cell lysates with 3xMBT, or with the binding-null D355N mutant as a negative control. Quantitative liquid chromatography and tandem mass spectrometry (LC-MS/MS) are then used to identify proteins that are specifically enriched by 3xMBT pull-down. The addition of a third isotopic label allows the comparison of protein lysine methylation between different biological conditions. Unlike most approaches, our strategy does not require a prior hypothesis of candidate methylated proteins, and it recognizes a wider range of methylated proteins than any available method using antibodies. Cells are prepared by growing in isotopic labeling medium for about 7 d; the process of enriching methylated proteins takes 3 d and analysis by LC-MS/MS takes another 1–2 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Huang, J. & Berger, S.L. The emerging field of dynamic lysine methylation of non-histone proteins. Curr. Opin. Genet. Dev. 18, 152–158 (2008).
Moore, K.E. et al. A general molecular affinity strategy for global detection and proteomic analysis of lysine methylation. Mol. Cell 50, 444–456 (2013).
Cao, X.J., Arnaudo, A.M. & Garcia, B.A. Large-scale global identification of protein lysine methylation in vivo. Epigenetics 8, 477–485 (2013).
Liu, H. et al. A method for systematic mapping of protein lysine methylation identifies functions for HP1β in DNA damage response. Mol. Cell 50, 723–735 (2013).
Ficarro, S.B. et al. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol. 20, 301–305 (2002).
Choudhary, C. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 (2009).
Kim, W. et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell 44, 325–340 (2011).
Li, H. et al. Structural basis for lower lysine methylation state-specific readout by MBT repeats of L3MBTL1 and an engineered PHD finger. Mol. Cell 28, 677–691 (2007).
Min, J. et al. L3MBTL1 recognition of mono- and dimethylated histones. Nat. Struct. Mol. Biol. 14, 1229–1230 (2007).
Ong, S. et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell Proteomics 1, 376–386 (2002).
Geiger, T., Cox, J., Ostasiewicz, P., Wisniewski, J.R. & Mann, M. Super-SILAC mix for quantitative proteomics of human tumor tissue. Nat. Methods 7, 383–385 (2010).
Vedadi, M. et al. A chemical probe selectively inhibits G9a and GLP methyltransferase activity in cells. Nat. Chem. Biol. 7, 566–574 (2011).
Porras-Yakushi, T.R., Whitelegge, J.P., Miranda, T.B. & Clarke, S. A novel SET domain methyltransferase modifies ribosomal protein Rpl23ab in yeast. J. Biol. Chem. 280, 34590–34598 (2005).
Levy, D. et al. A proteomic approach for the identification of novel lysine methyltransferase substrates. Epigenetics Chromatin 4, 19 (2011).
Ong, S.E., Mittler, G. & Mann, M. Identifying and quantifying in vivo methylation sites by heavy methyl SILAC. Nat. Methods 1, 119–126 (2004).
Bremang, M. et al. Mass spectrometry-based identification and characterisation of lysine and arginine methylation in the human proteome. Mol. Biosyst. 9, 2231–2247 (2013).
Ong, S.E. & Mann, M. A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC). Nat. Protoc. 1, 2650–2660 (2006).
Tachibana, M., Sugimoto, K., Fukushima, T. & Shinkai, Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J. Biol. Chem. 276, 25309–25317 (2001).
Zhang, X., Wen, H. & Shi, X. Lysine methylation: beyond histones. Acta Biochim. Biophys. Sin. 44, 14–27 (2012).
Swaney, D.L., Wenger, C.D. & Coon, J.J. Value of using multiple proteases for large-scale mass spectrometry-based proteomics. J. Proteome Res. 9, 1323–1329 (2010).
Kubota, T., Stead, D.A., Hiraga, S., ten Have, S. & Donaldson, A.D. Quantitative proteomic analysis of yeast DNA replication proteins. Methods 57, 196–202 (2012).
Ong, S.E. & Mann, M. Stable isotope labeling by amino acids in cell culture for quantitative proteomics. Methods Mol. Biol. 359, 37–52 (2007).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Perkins, D.N., Pappin, D.J., Creasy, D.M. & Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 (1999).
Eng, J.K., McCormack, A.L. & Yates, J.R.I. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5, 976–989 (1994).
Porras-Yakushi, T.R., Whitelegge, J.P. & Clarke, S. A novel SET domain methyltransferase in yeast: Rkm2-dependent trimethylation of ribosomal protein L12ab at lysine 10. J. Biol. Chem. 281, 35835–35845 (2006).
Vizcaíno, J.A. et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 41, D1063–D1069 (2013).
Acknowledgements
This work was supported in part by a grant from the US National Institutes of Health (NIH) to O.G. (R01 GM079641). S.M.C. was supported by an American Cancer Society Illinois Division Postdoctoral Fellowship award 123711-PF-13-093-01-TBE. K.E.M. was supported by a Hubert Shaw and Sandra Lui Stanford Graduate Fellowship. This material is based on work supported by the National Science Foundation Graduate Research Fellowship under grant no. DGE-1147470 to K.E.M. G.M.M. was supported by the Generalitat de Catalunya Beatriu de Pinos award BP-A 2010. O.G. is a recipient of an Ellison Senior Scholar in Aging Award. We thank the PRIDE team for assistance in making mass spectrometry data available.
Author information
Authors and Affiliations
Contributions
S.M.C. and K.E.M. developed the 3xMBT pull-down protocol. S.M.C. optimized the protocol for mass spectrometry. E.M.G. conducted the Rkm1 experiment. G.M.M. generated SILAC yeast strains and adapted the protocol to yeast. S.M.C. and K.E.M. wrote the protocol. O.G. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
O.G. is a cofounder of EpiCypher, Inc. Stanford University has submitted a provisional US patent describing the use of the 3xMBT domain as an affinity reagent.
Supplementary information
Supplementary Table 1
Protein identification and SILAC data from 3xMBT pull-down of lysate from yeast Δrkm1 and wild-type cells. (XLSX 17 kb)
Rights and permissions
About this article
Cite this article
Carlson, S., Moore, K., Green, E. et al. Proteome-wide enrichment of proteins modified by lysine methylation. Nat Protoc 9, 37–50 (2014). https://doi.org/10.1038/nprot.2013.164
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.164
This article is cited by
-
Global lysine methylome profiling using systematically characterized affinity reagents
Scientific Reports (2023)
-
Tumor-suppressive functions of protein lysine methyltransferases
Experimental & Molecular Medicine (2023)
-
DNA and histone modifications as potent diagnostic and therapeutic targets to advance non-small cell lung cancer management from the perspective of 3P medicine
EPMA Journal (2022)
-
Cell-Wide Survey of Amide-Bonded Lysine Modifications by Using Deacetylase CobB
Biological Procedures Online (2019)
-
Lysine methylation signaling of non-histone proteins in the nucleus
Cellular and Molecular Life Sciences (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.