Abstract
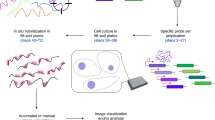
We present a protocol for measuring the absolute number of mRNA molecules from a gene of interest in individual, chemically fixed Escherichia coli cells. A set of fluorescently labeled oligonucleotide probes is hybridized to the target mRNA, such that each mRNA molecule is decorated by a known number of fluorescent dyes. Cells are then imaged using fluorescence microscopy. The copy number of the target mRNA is estimated from the total intensity of fluorescent foci in the cell, rather than from counting discrete 'spots' as in other currently available protocols. Image analysis is performed using an automated algorithm. The measured mRNA copy number distribution obtained from many individual cells can be used to extract the parameters of stochastic gene activity, namely the frequency and size of transcription bursts from the gene of interest. The experimental procedure takes 2 d, with another 2–3 d typically required for image and data analysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
19 August 2015
In the version of this article initially published, a component (40 µl of 50 mg ml-1 BSA) was erroneously omitted from the 'Hybridization solution' recipe in the Reagent Setup section. The error has been corrected in the HTML and PDF versions of the article.
References
Femino, A.M., Fay, F.S., Fogarty, K. & Singer, R.H. Visualization of single RNA transcripts in situ. Science 280, 585–590 (1998).
Raj, A., Peskin, C.S., Tranchina, D., Vargas, D.Y. & Tyagi, S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 4, e309 (2006).
Zenklusen, D., Larson, D.R. & Singer, R.H. Single-RNA counting reveals alternative modes of gene expression in yeast. Nat. Struct. Mol. Biol. 15, 1263–1271 (2008).
Raj, A., van den Bogaard, P., Rifkin, S.A., van Oudenaarden, A. & Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877–879 (2008).
Taniguchi, Y. et al. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science 329, 533–538 (2010).
Trcek, T. et al. Single-mRNA counting using fluorescent in situ hybridization in budding yeast. Nat. Protoc. 7, 408–419 (2012).
Zenklusen, D. & Singer, R.H. Analyzing mRNA expression using single mRNA resolution fluorescent in situ hybridization. Methods Enzymol. 470, 641–659 (2010).
Zong, C., So, L.H., Sepulveda, L.A., Skinner, S.O. & Golding, I. Lysogen stability is determined by the frequency of activity bursts from the fate-determining gene. Mol. Syst. Biol. 6, 440 (2010).
So, L.H. et al. General properties of transcriptional time series in Escherichia coli. Nat. Genet. 43, 554–560 (2011).
Golding, I., Paulsson, J., Zawilski, S.M. & Cox, E.C. Real-time kinetics of gene activity in individual bacteria. Cell 123, 1025–1036 (2005).
Thompson, R.E., Larson, D.R. & Webb, W.W. Precise nanometer localization analysis for individual fluorescent probes. Biophys. J. 82, 2775–2283 (2002).
Lubeck, E. & Cai, L. Single-cell systems biology by super-resolution imaging and combinatorial labeling. Nat. Methods 9, 743–748 (2012).
Golding, I. & Cox, E.C. Chapter 8: Spatiotemporal dynamics in bacterial cells: real-time studies with single-event resolution. Methods Cell Biol. 89, 223–251 (2008).
Maamar, H., Raj, A. & Dubnau, D. Noise in gene expression determines cell fate in Bacillus subtilis. Science 317, 526–529 (2007).
Kafri, R. et al. Dynamics extracted from fixed cells reveal feedback linking cell growth to cell cycle. Nature 494, 480–483 (2013).
Peccoud, J. & Ycart, B. Markovian modeling of gene-product synthesis. Theor. Popul. Biol. 48, 222–234 (1995).
Shahrezaei, V. & Swain, P.S. Analytical distributions for stochastic gene expression. Proc. Natl. Acad. Sci. USA 105, 17256–17261 (2008).
Levsky, J.M., Shenoy, S.M., Pezo, R.C. & Singer, R.H. Single-cell gene expression profiling. Science 297, 836–840 (2002).
Long, R.M. et al. Mating type switching in yeast controlled by asymmetric localization of ASH1 mRNA. Science 277, 383–387 (1997).
Golding, I. & Cox, E.C. RNA dynamics in live Escherichia coli cells. Proc. Natl. Acad. Sci. USA 101, 11310–11315 (2004).
Montero Llopis, P. et al. Spatial organization of the flow of genetic information in bacteria. Nature 466, 77–81 (2010).
Nevo-Dinur, K., Nussbaum-Shochat, A., Ben-Yehuda, S. & Amster-Choder, O. Translation-independent localization of mRNA in E. coli. Science 331, 1081–1084 (2011).
Bakshi, S., Siryaporn, A., Goulian, M. & Weisshaar, J.C. Superresolution imaging of ribosomes and RNA polymerase in live Escherichia coli cells. Mol. Microbiol. 85, 21–38 (2012).
Kuhlman, T.E. & Cox, E.C. Gene location and DNA density determine transcription factor distributions in Escherichia coli. Mol. Syst. Biol. 8, 610 (2012).
Phillips, R., Kondev, J., Theriot, J. & Garcia, H. Physical Biology of the Cell (Garland Science, 2012).
Yu, J., Xiao, J., Ren, X., Lao, K. & Xie, X.S. Probing gene expression in live cells, one protein molecule at a time. Science 311, 1600–1603 (2006).
Hebenstreit, D. et al. RNA sequencing reveals two major classes of gene expression levels in metazoan cells. Mol. Syst. Biol. 7, 497 (2011).
Bertrand, E. et al. Localization of ASH1 mRNA particles in living yeast. Mol. Cell 2, 437–445 (1998).
Fusco, D. et al. Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr. Biol. 13, 161–167 (2003).
Young, J.W. et al. Measuring single-cell gene expression dynamics in bacteria using fluorescence time-lapse microscopy. Nat. Protoc. 7, 80–88 (2012).
Cohen, A.A. et al. Dynamic proteomics of individual cancer cells in response to a drug. Science 322, 1511–1516 (2008).
Geva-Zatorsky, N. et al. Protein dynamics in drug combinations: a linear superposition of individual-drug responses. Cell 140, 643–651 (2010).
Neidhardt, F.C. (ed.) Escherichia coli and Salmonella: Cellular and Molecular Biology (ASM Press, 1996).
Bates, D. & Kleckner, N. Chromosome and replisome dynamics in E. coli: loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell 121, 899–911 (2005).
Kuhlman, T., Zhang, Z., Saier, M.H. Jr. & Hwa, T. Combinatorial transcriptional control of the lactose operon of Escherichia coli. Proc. Natl. Acad. Sci. USA 104, 6043–6048 (2007).
Yakovleva, G.M., Kim, S.K. & Wanner, B.L. Phosphate-independent expression of the carbon-phosphorus lyase activity of Escherichia coli. Appl. Microbiol. Biotechnol. 49, 573–588 (1998).
Raj, A. & Tyagi, S. Detection of individual endogenous RNA transcripts in situ using multiple singly labeled probes. Methods Enzymol. 472, 365–386 (2010).
Batish, M., Raj, A. & Tyagi, S. Single molecule imaging of RNA in situ. Methods Mol. Biol. 714, 3–13 (2010).
Sliusarenko, O., Heinritz, J., Emonet, T. & Jacobs-Wagner, C. High-throughput, subpixel precision analysis of bacterial morphogenesis and intracellular spatio-temporal dynamics. Mol. Microbiol. 80, 612–627 (2011).
Paulsson, J. & Ehrenberg, M. Random signal fluctuations can reduce random fluctuations in regulated components of chemical regulatory networks. Phys. Rev. Lett. 84, 5447–5450 (2000).
Acknowledgements
C. Zong and L.-H. So first introduced the smFISH protocol in our lab. We thank A. Raj, R. Singer and L. Cai for generous advice. We thank all members of the Golding lab for providing help with experiments. The Schnitzcells software was kindly provided by M. Elowitz (California Institute of Technology). Work in the Golding lab was supported by the US National Institutes of Health grant no. R01 GM082837, US National Science Foundation grant nos. 082265 (Physics Frontiers Center: Center for the Physics of Living Cells) and PHY-1147498 (CAREER), Human Frontier Science Program grant no. RGY 70/2008 and Welch Foundation grant no. Q-1759.
Author information
Authors and Affiliations
Contributions
I.G. supervised the project. S.O.S., L.A.S. and H.X. developed the protocol. S.O.S., L.A.S. and I.G. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Skinner, S., Sepúlveda, L., Xu, H. et al. Measuring mRNA copy number in individual Escherichia coli cells using single-molecule fluorescent in situ hybridization. Nat Protoc 8, 1100–1113 (2013). https://doi.org/10.1038/nprot.2013.066
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.066
This article is cited by
-
Gene expression model inference from snapshot RNA data using Bayesian non-parametrics
Nature Computational Science (2023)
-
Porin-independent accumulation in Pseudomonas enables antibiotic discovery
Nature (2023)
-
Previously uncharacterized rectangular bacterial structures in the dolphin mouth
Nature Communications (2023)
-
Small protein modules dictate prophage fates during polylysogeny
Nature (2023)
-
Targeting nucleic acid phase transitions as a mechanism of action for antimicrobial peptides
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.