Abstract
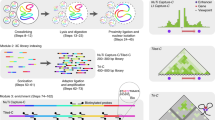
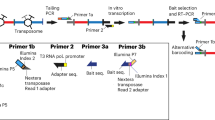
Chromosome conformation capture (3C) technology is a powerful and increasingly popular tool for analyzing the spatial organization of genomes. Several 3C variants have been developed (e.g., 4C, 5C, ChIA-PET, Hi-C), allowing large-scale mapping of long-range genomic interactions. Here we describe multiplexed 3C sequencing (3C-seq), a 4C variant coupled to next-generation sequencing, allowing genome-scale detection of long-range interactions with candidate regions. Compared with several other available techniques, 3C-seq offers a superior resolution (typically single restriction fragment resolution; approximately 1–8 kb on average) and can be applied in a semi-high-throughput fashion. It allows the assessment of long-range interactions of up to 192 genes or regions of interest in parallel by multiplexing library sequencing. This renders multiplexed 3C-seq an inexpensive, quick (total hands-on time of 2 weeks) and efficient method that is ideal for the in-depth analysis of complex genetic loci. The preparation of multiplexed 3C-seq libraries can be performed by any investigator with basic skills in molecular biology techniques. Data analysis requires basic expertise in bioinformatics and in Linux and Python environments. The protocol describes all materials, critical steps and bioinformatics tools required for successful application of 3C-seq technology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dixon, J.R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012).
Nora, E.P. et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385 (2012).
Sanyal, A., Lajoie, B.R., Jain, G. & Dekker, J. The long-range interaction landscape of gene promoters. Nature 489, 109–113 (2012).
Splinter, E. & de Laat, W. The complex transcription regulatory landscape of our genome: control in three dimensions. EMBO J. 30, 4345–4355 (2011).
Bulger, M. & Groudine, M. Functional and mechanistic diversity of distal transcription enhancers. Cell 144, 327–339 (2011).
Ong, C.T. & Corces, V.G. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat. Rev. Genet. 12, 283–293 (2011).
Stadhouders, R. et al. Transcription regulation by distal enhancers: who's in the loop? Transcription 3, 181–186 (2012).
Dekker, J., Rippe, K., Dekker, M. & Kleckner, N. Capturing chromosome conformation. Science 295, 1306–1311 (2002).
Gondor, A., Rougier, C. & Ohlsson, R. High-resolution circular chromosome conformation capture assay. Nat. Protoc. 3, 303–313 (2008).
Sexton, T. et al. Sensitive detection of chromatin coassociations using enhanced chromosome conformation capture on chip. Nat. Protoc. 7, 1335–1350 (2012).
Simonis, M. et al. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C). Nat. Genet. 38, 1348–1354 (2006).
van de Werken, H.J. et al. Robust 4C-seq data analysis to screen for regulatory DNA interactions. Nat. Methods 9, 969–972 (2012).
Dostie, J. & Dekker, J. Mapping networks of physical interactions between genomic elements using 5C technology. Nat. Protoc. 2, 988–1002 (2007).
Fullwood, M.J. et al. An oestrogen-receptor-α-bound human chromatin interactome. Nature 462, 58–64 (2009).
Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293 (2009).
Soler, E. et al. The genome-wide dynamics of the binding of Ldb1 complexes during erythroid differentiation. Genes Dev. 24, 277–289 (2010).
Stadhouders, R. et al. Dynamic long-range chromatin interactions control Myb proto-oncogene transcription during erythroid development. EMBO J. 31, 986–999 (2012).
Ribeiro de Almeida, C. et al. The DNA-binding protein CTCF limits proximal Vκ recombination and restricts κ enhancer interactions to the immunoglobulin κ light chain locus. Immunity 35, 501–513 (2011).
Hagege, H. et al. Quantitative analysis of chromosome conformation capture assays (3C-qPCR). Nat. Protoc. 2, 1722–1733 (2007).
Naumova, N., Smith, E.M., Zhan, Y. & Dekker, J. Analysis of long-range chromatin interactions using chromosome conformation capture. Methods (2012).
Ecker, J.R. et al. Genomics: ENCODE explained. Nature 489, 52–55 (2012).
Dostie, J. & Bickmore, W.A. Chromosome organization in the nucleus—charting new territory across the Hi-Cs. Curr. Opin. Genet Dev. 22, 125–131 (2012).
Comet, I., Schuettengruber, B., Sexton, T. & Cavalli, G. A chromatin insulator driving three-dimensional Polycomb response element (PRE) contacts and Polycomb association with the chromatin fiber. Proc. Natl. Acad. Sci. USA 108, 2294–2299 (2011).
Jing, H. et al. Exchange of GATA factors mediates transitions in looped chromatin organization at a developmentally regulated gene locus. Mol. Cell 29, 232–242 (2008).
Rippe, K., von Hippel, P.H. & Langowski, J. Action at a distance: DNA-looping and initiation of transcription. Trends Biochem. Sci. 20, 500–506 (1995).
Dekker, J. The three 'C' s of chromosome conformation capture: controls, controls, controls. Nat. Methods 3, 17–21 (2006).
Brouwer, R.W., van den Hout, M.C., Grosveld, F.G. & van Ijcken, W.F. NARWHAL, a primary analysis pipeline for NGS data. Bioinformatics 28, 284–285 (2012).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Quinlan, A.R. & Hall, I.M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
van de Werken, H.J. et al. 4C technology: protocols and data analysis. Methods Enzymol. 513, 89–112 (2012).
Hakim, O. et al. DNA damage defines sites of recurrent chromosomal translocations in B lymphocytes. Nature 484, 69–74 (2012).
Zhang, Y. et al. Spatial organization of the mouse genome and its role in recurrent chromosomal translocations. Cell 148, 908–921 (2012).
Visser, M., Kayser, M. & Palstra, R.J. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Res. 22, 446–455 (2012).
Lin, Y.C. et al. Global changes in the nuclear positioning of genes and intra- and interdomain genomic interactions that orchestrate B cell fate. Nat. Immunol. 13, 1196–1204 (2012).
Acknowledgements
We thank A. van der Sloot, Z. Ozgur, E. Oole, M. van den Hout, F. Sleutels, S.Thongjuea and B. Lenhard for their help in sample processing, bioinformatics pipeline development and data analysis. R.S. received support from the Royal Netherlands Academy of Arts and Sciences (KNAW). P.K. was supported by grants from ERASysBio+/FP7 (project no. 93511024). E.S. was supported by grants from the Dutch Cancer Genomics Center, the Netherlands Genomics Initiative (project no. 40-41009-98-9082) and the French Alternative Energies and Atomic Energy Commission (CEA). This work was supported by the EU-FP7 Eutracc consortium.
Author information
Authors and Affiliations
Contributions
R.S. and R.-J.P. adapted and optimized the protocol and library preparation for Illumina sequencing. R.S., P.K., A.v.d.H. and J.Z. used, developed and troubleshot the technique. C.K. optimized procedures for library sequencing, and R.B. developed the informatics pipeline for data processing and analysis. W.v.I., F.G., K.S.W. and E.S. supervised the projects, and participated in technology design and discussions. R.S., P.K., R.B., W.v.I., F.G., K.S.W. and E.S. drafted the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Data
Scripts to analyze 3C-seq data. Within this supplementary archive, 4 python files are present that are used to analyze 3C-seq data. The findSequence.py and the regionsBetween.py files are used to generate a restriction map of the genome. The alignCounter.py script determines how many reads align to each of the restriction fragments. To run the alignCounter.py script the Pysam libraries should be installed on the system (see Materials). The libutils.py script is a shared library that should be placed in the same directory as the other py files. This library contains shared functionality between the 3 executable scripts. (ZIP 5 kb)
Supplementary Table 1
NARWHAL sample sheet. This file contains an example NARWHAL sample sheet that can be used in the primary data analysis. It serves to illustrate the specific fields that should be set in the primary data analysis procedure (Steps 65-79). (TXT 1 kb)
Rights and permissions
About this article
Cite this article
Stadhouders, R., Kolovos, P., Brouwer, R. et al. Multiplexed chromosome conformation capture sequencing for rapid genome-scale high-resolution detection of long-range chromatin interactions. Nat Protoc 8, 509–524 (2013). https://doi.org/10.1038/nprot.2013.018
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.018
This article is cited by
-
JMJD3 intrinsically disordered region links the 3D-genome structure to TGFβ-dependent transcription activation
Nature Communications (2022)
-
HOXBLINC long non-coding RNA activation promotes leukemogenesis in NPM1-mutant acute myeloid leukemia
Nature Communications (2021)
-
DNA methylation changes during long-term in vitro cell culture are caused by epigenetic drift
Communications Biology (2021)
-
TNF-α-producing macrophages determine subtype identity and prognosis via AP1 enhancer reprogramming in pancreatic cancer
Nature Cancer (2021)
-
A 16q22.1 variant confers susceptibility to colorectal cancer as a distal regulator of ZFP90
Oncogene (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.