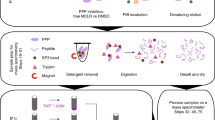
Abstract
Characterizing the components of GW/processing bodies is key to elucidating RNA interference and messenger RNA processing pathways. This protocol addresses challenges in isolating a low-abundance protein GW182 and GW body (GWB)-associated proteins by building on previous reports that used polyclonal sera containing autoantibodies to GW/P body components. This protocol uses commercially available monoclonal antibodies to GW182 that are covalently coupled to Protein A or G sepharose beads and then used to immunoprecipitate GW182 and associated proteins from cell extracts. Immunoprecipitates are separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes and probed by western blot with antibodies directed to proteins of interest. This protocol, which is expected to take 4–5 d, provides a biochemical approach for detecting GW182 and associated proteins in biological samples and thus facilitates the elucidation of the diverse functions of GWBs. It is expected that this protocol can be adapted to the detection of other RNA-binding complexes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eystathioy, T. et al. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell 13, 1338–1351 (2002).
Sheth, U. & Parker, R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300, 805–808 (2003).
Eulalio, A., Behm-Ansmant, I. & Izaurralde, E. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8, 9–22 (2007).
Mello, C.C. & Conte, D. Jr. Revealing the world of RNA interference. Nature 431, 338–342 (2004).
Meister, G. & Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 431, 343–349 (2004).
Eystathioy, T. et al. The GW182 protein co-localizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA 9, 1171–1173 (2003).
Anderson, P. & Kedersha, N. RNA granules. J. Cell Biol. 172, 803–808 (2006).
Jakymiw, A. et al. The role of GW/P bodies in RNA processing and silencing. J. Cell Sci. 120, 1317–1323 (2007).
Fillman, C. & Lykke-Andersen, J. RNA decapping inside and outside of processing bodies. Curr. Opin. Cell Biol. 17, 326–331 (2005).
Andrei, M.A. et al. A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA 11, 717–727 (2005).
Lee, H.C., Cho, H. & Kim, Y.K. Ectopic expression of eIF4E-transporter triggers the movement of eIF4E into P-bodies, inhibiting steady-state translation but not the pioneer round of translation. Biochem. Biophys. Res. Commun. 369, 1160–1165 (2008).
Lian, S. et al. Small interfering RNAs-mediated silencing induces target-dependent assembly of GW bodies. Mol. Biol. Cell 18, 3375–3387 (2007).
Filipowicz, W., Bhattacharyya, S.N. & Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114 (2008).
Lau, N.C. et al. Characterization of the piRNA complex from rat testes. Science 313, 363–367 (2006).
Buchan, J.R., Muhlrad, D. & Parker, R. P bodies promote stress granule assembly in Saccharomyces cerevisiae . J. Cell Biol. 183, 441–455 (2008).
Anderson, P. & Kedersha, N. Stressful initiations. J. Cell Sci. 115, 3227–3234 (2002).
Kedersha, N. & Anderson, P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans. 30, 963–969 (2002).
Kedersha, N. et al. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169, 871–884 (2005).
Kedersha, N. & Anderson, P. Mammalian stress granules and processing bodies. Methods Enzymol. 431, 61–81 (2007).
Anderson, P. & Kedersha, N. Stress granules: the Tao of RNA triage. Trends Biochem. Sci. 33, 141–150 (2008).
Moser, J.J., Eystathioy, T., Chan, E.K.L. & Fritzler, M.J. Markers of mRNA stabilization and degradation, and RNAi within astrocytoma GW bodies. J. Neurosci. Res. 85, 3619–3631 (2007).
Li, S. et al. Identification of GW182 and its novel isoform TNGW1 as translational repressors in Ago-2-mediated silencing. J. Cell Sci. 121, 4134–4144 (2008).
Zeng, F. et al. A protocol for PAIR: PNA-assisted identification of RNA binding proteins in living cells. Nat. Protoc. 1, 920–927 (2006).
Tenenbaum, S.A., Carson, C.C., Lager, P.J. & Keene, J.D. Identifying mRNA subsets in messenger ribonucleoprotein complexes by using cDNA arrays. Proc. Natl. Acad. Sci. USA 97, 14085–14090 (2000).
Ikeda, K. et al. Detection of the argonaute protein Ago2 and microRNAs in the RNA induced silencing complex (RISC) using a monoclonal antibody. J. Immunol. Methods 317, 38–44 (2006).
Eystathioy, T. et al. A panel of monoclonal antibodies to cytoplasmic GW bodies and the mRNA binding protein GW182. Hybridoma Hybridomics 22, 79–86 (2003).
Eystathioy, T. et al. Clinical and serological associations of autoantibodies to a novel cytoplasmic autoantigen, GW182 and GW bodies. J. Mol. Med. 81, 811–818 (2003).
Jakymiw, A. et al. Disruption of GW bodies impairs mammalian mRNA interference. Nat. Cell Biol. 7, 1167–1174 (2005).
Liu, J. et al. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 7, 1161–1166 (2005).
Liu, J., Valencia-Sanchez, M.A., Hannon, G.J. & Parker, R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell Biol. 7, 719–723 (2005).
Pillai, R.S. et al. Inhibition of translational initiation by Let-7 microRNA in human cells. Science 309, 1573–1576 (2005).
Rehwinkel, J., Behm-Ansmant, I., Gatfield, D. & Izaurralde, E. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA 11, 1640–1647 (2005).
Sen, G.L. & Blau, H.M. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 7, 633–636 (2005).
Eulalio, A., Huntzinger, E. & Izaurralde, E. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat. Struct. Mol Biol. 15, 346–353 (2008).
Acknowledgements
This work was supported in part by the Canadian Institutes for Health Research Grant MOP-57674 and NIH Grant AI47859. M.J.F. holds the Arthritis Society Chair. J.J.M. is supported by a CIHR Doctoral Research Award in the Area of Clinical Research and by an Alberta Heritage Foundation for Medical Research Studentship Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moser, J., Chan, E. & Fritzler, M. Optimization of immunoprecipitation–western blot analysis in detecting GW182-associated components of GW/P bodies. Nat Protoc 4, 674–685 (2009). https://doi.org/10.1038/nprot.2009.34
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.34
This article is cited by
-
Application of smart solid lipid nanoparticles to enhance the efficacy of 5-fluorouracil in the treatment of colorectal cancer
Scientific Reports (2020)
-
Antibody-driven capture of synaptic vesicle proteins on the plasma membrane enables the analysis of their interactions with other synaptic proteins
Scientific Reports (2019)
-
Characterization of Cerebrospinal Fluid BACE1 Species
Molecular Neurobiology (2019)
-
Biophysical Characterization of SG2NA Variants and their Interaction with DJ-1 and Calmodulin in vitro
Cell Biochemistry and Biophysics (2018)
-
C-terminal fragments of the amyloid precursor protein in cerebrospinal fluid as potential biomarkers for Alzheimer disease
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.