Abstract
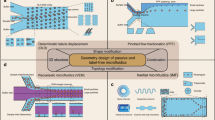
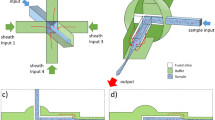
The anisotropic nanofluidic-filter (nanofilter) array (ANA) is a unique molecular-sieving structure for separating biomolecules. In this protocol we describe the fabrication of planar and vertical ANA chips and how to perform continuous-flow bioseparation using them. This protocol is most useful for bioengineers who are interested in developing automated multistep chip-based bioanalysis systems and assumes previous cleanroom microfabrication knowledge. The ANA consists of a two-dimensional periodic nanofilter array, and the designed structural anisotropy of ANA causes different-sized or charged biomolecules to follow distinct trajectories under applied electric fields, leading to efficient continuous-flow separation. Using microfluidic channels surrounding the ANA, the fractionated biomolecule streams are collected and routed to different fluid channels or reservoirs for convenient sample recovery and downstream bioanalysis. The ANA is physically robust and can be reused repeatedly. Compared with the conventional gel-based separation techniques, ANA offers the potential for faster separation, higher throughput and more convenient sample recovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Goodsell, D.S. Bionanotechnology, Lessons from Nature (Wiley-Liss, Hoboken, New Jersey, 2004).
Jain, K.K. Nanobiotechnology in Molecular Diagnostics, Current Techniques and Applications (Horizontal Bioscience, Norfolk, UK, 2006).
Chu, S. Biology and polymer physics at the single-molecule level. Philos. Trans. R. Soc. London Ser. A-Math. Phys. Eng. Sci. 361, 689–693 (2003).
Fortina, P., Kricka, L.J., Surrey, S. & Grodzinski, P. Nanobiotechnology: the promise and reality of new approaches to molecular recognition. Trends Biotechnol. 23, 168–173 (2005).
Hinterdorfer, P. & Dufrêne, Y.F. Detection and localization of single molecular recognition events using atomic force microscopy. Nature Methods 3, 347–355 (2006).
Eijkel, J.C.T. & van den Berg, A. Nanofluidics: what is it and what can we expect from it? Microfluid. Nanofluid. 1, 249–267 (2005).
Craighead, H. Future lab-on-a-chip technologies for interrogating individual molecules. Nature 442, 387–393 (2006).
Tegenfeldt, J.O et al. Micro- and nanofluidics for DNA analysis. Anal. Bioanal. Chem. 378, 1678–1692 (2004).
Han, J., Fu, J. & Schoch, R.B. Molecular sieving using nanofilters: past, present and future. Lab Chip 8, 23–33 (2008).
Fu, J., Mao, P. & Han, J. Artificial molecular sieves and filters: a new paradigm for biomolecule separation. Trends Biotech. 26, 311–320 (2008).
Slater, G.W. Theory of DNA electrophoresis: a look at some current challenges. Electrophoresis 21, 3873–3887 (2000).
Whitesides, G.M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
El-Ali, J., Sorger, P.K. & Jensen, K.F. Cells on chips. Nature 442, 403–411 (2006).
Han, J. & Craighead, H.G. Separation of long DNA molecules in a microfabricated entropic trap array. Science 288, 1026–1029 (2000).
Huang, L.R. et al. A DNA prism: high-speed continuous fractionation of large DNA molecules. Nature Biotech. 20, 1048–1051 (2002).
Baba, M. et al. DNA size separation using artificially nanostructured matrix. Appl. Phys. Lett. 83, 1468–1470 (2003).
Kaji, N. et al. Separation of long DNA molecules by quartz nanopillar chips under a direct current electric field. Anal. Chem. 76, 15–22 (2004).
Huang, L.R., Cox, E.C., Austin, R.H. & Sturm, J.C . Continuous particle separation through deterministic lateral displacement. Science 304, 987–990 (2004).
Fu, J., Schoch, R.B., Bow, H., Stevens, A.L., Tannenbaum, S.R. & Han, J. A patterned anisotropic nanofluidic sieving structure for continuous-flow separation of DNA and proteins. Nature Nanotech. 2, 121–128 (2007).
Mao, P. & Han, J. Massively-parallel ultra-high-aspect-ratio nanochannels as mesoporous membranes. Lab Chip 9, 586–591 (2009).
Scopes, R.K. Protein Purification, Principles and Practice, ed. 3 (Springer-Verlag, New York, 1993).
Giddings, J.C. Dynamics of Chromatography. Part 1. Principles and Theory (Marcel Dekker, New York, 1965).
Yao, G. et al. SDS capillary gel electrophoresis of proteins in microfabricated channels. Proc. Natl. Acad. Sci. USA 96, 5372–5377 (1999).
Callewaert, N. et al. Total serum protein N-glycome profiling on a capillary electrophoresis-microfluidics platform. Electrophoresis 25, 3128–3131 (2004).
Turner, S.W., Perez, A.M., Lopez, A. & Craighead, H.G. Monolithic nanofluid sieving structures for DNA manipulation. J. Vac. Sci. Technol. B 16, 3835–3840 (1998).
Cao, H. et al. Fabrication of 10 nm enclosed nanofluidic channels. Appl. Phys. Lett. 81, 174–176 (2002).
Fu, J., Mao, P. & Han, J. Nanofilter array chip for fast gel-free biomolecule separation. Appl. Phys. Lett. 87, 263902 (2005).
Fu, J., Yoo, J. & Han, J. Molecular sieving in periodic free-energy landscapes created by patterned nanofilter arrays. Phys. Rev. Lett. 97, 018103 (2006).
Eijkel, J.C.T. & van den Berg, A. Nanotechnology for membranes, filters and sieves. Lab Chip 6, 19–23 (2006).
Austin, R. Nanofluidics: a fork in the nano-road. Nature Nanotech. 2, 121–128 (2007).
Pamme, N. Continuous flow separations in microfluidic devices. Lab Chip 7, 1644–1659 (2007).
Wulfkuhle, J.D., Liotta, L.A. & Petricoin, E.F. Proteomic applications for the early detection of cancer. Nat. Rev. Cancer 3, 267–275 (2003).
Righetti, P.G., Castagna, A., Herbert, B., Reymond, F. & Rossier, J.S. Prefractionation techniques in proteome analysis. Proteomics 3, 1397–1407 (2003).
Mao, P. & Han, J. Fabrication and characterization of 20-nm nanofluidic channels by glass-glass and glass-silicon bonding. Lab Chip 5, 837–844 (2005).
Bow, H., Fu, J. & Han, J. Decreasing effective nanofluidic filter size by modulating electrical double layers: separation enhancement in microfabricated nanofluidic filters. Electrophoresis 29, 4646–4651 (2008).
Huang, L.R. et al. Generation of large-area tunable uniform electric fields in microfluid arrays for rapid DNA separation. Tech. Dig. Int. Elect. Dev. Mtg. 363–366 (2002).
Acknowledgements
The authors acknowledge financial support from the National Institute of Health (EB005743), Korea Institute of Science and Technology–Intelligent Microsystems Center (KIST-IMC), and the Singapore-MIT Alliance (SMA-II, CE program). We also thank J. Yoo for his contribution in the experimental setup and H. Bow and S. Reto for helpful discussions. The MIT Microsystems Technology Laboratories is acknowledged for support in microfabrication.
Author information
Authors and Affiliations
Contributions
J.F., P.M. and J.H. conceived and designed the ANA chips. J.F. and P.M. fabricated the ANA chips. J.F. and P.M. designed and performed experiments, and analyzed data. J.F. and P.M. wrote manuscript.
Corresponding author
Supplementary information
Supplementary Figure 1
Cleanroom equipments used for fabrication of the ANA structures (PDF 509 kb)
Rights and permissions
About this article
Cite this article
Fu, J., Mao, P. & Han, J. Continuous-flow bioseparation using microfabricated anisotropic nanofluidic sieving structures. Nat Protoc 4, 1681–1698 (2009). https://doi.org/10.1038/nprot.2009.176
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.176
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.