Abstract
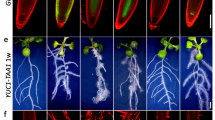
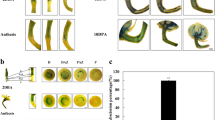
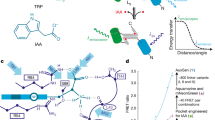
This protocol allows the measurement of auxin transport in roots, hypocotyls and inflorescences of Arabidopsis thaliana plants by examining transport of radiolabeled auxin or movement of an auxin-induced gene expression signal. The protocol contains four stages: seedling growth, auxin application, a transport period of variable length, and quantification of auxin movement or reporter expression. Beyond the time for plant growth, the transport assay can be completed within 4–18 h. Auxin is applied to seedlings in agar cylinders or droplets, which does not require specialized liquid-handling equipment or micromanipulators, in contrast with methods that apply auxin in liquid droplets. Spatial control of auxin application is reduced, but this method has the advantages of being technically more feasible for most laboratories and allowing agar containing radioactive auxin to be removed for pulse chase assays that determine transport rates. These methods allow investigation of genetic and environmental factors that control auxin transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Blakeslee, J.J., Peer, W.A. & Murphy, A.S. Auxin transport. Curr. Opin. Plant Biol. 8, 494–500 (2005).
Sánchez-Bravo, J., Ortuño, A.M., Botía, J.M., Acosta, M. & Sabater, F. The decrease in auxin polar transport down the lupin hypocotyl could produce the indole-3-acetic acid distribution responsible for the elongation growth pattern. Plant Physiol. 99, 108–114 (1992).
Bhalerao, R.P. & Bennett, M.J. The case for morphogens in plants. Nat. Cell Biol. 5, 939–943 (2003).
Casimiro, I. et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13, 843–852 (2001).
Muday, G.K. & DeLong, A. Polar auxin transport: controlling where and how much. Trends Plant Sci. 6, 535–542 (2001).
Goldsmith, M.H.M. The polar transport of auxin. Ann. Rev. Plant Physiol. 28, 439–478 (1977).
Okada, K., Ueda, J., Komaki, M.K., Bell, C.J. & Shimura, Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3, 677–684 (1991).
Mitchell, E.K. & Davies, P.J. Evidence for three different systems of movement of indoleacetic acid in intact roots of Phaseolus coccineus . Physiol Plant. 33, 290–294 (1975).
Tsurumi, S. & Ohwaki, Y. Transport of 14C-labeled indoleacetic acid in Vicia root segments. Plant Cell Physiol. 19, 1195–1206 (1978).
Rashotte, A.M., Poupart, J., Waddell, C.S. & Muday, G.K. Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis . Plant Physiol. 133, 761–772 (2003).
Rashotte, A.M., Brady, S.R., Reed, R.C., Ante, S.J. & Muday, G.K. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis . Plant Physiol. 122, 481–490 (2000).
Rashotte, A.M., DeLong, A. & Muday, G.K. Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13, 1683–1697 (2001).
Kramer, E.M. PIN and AUX/LAX proteins: their role in auxin accumulation. Trends Plant Sci. 9, 578–582 (2004).
Leyser, O. Dynamic integration of auxin transport and signalling. Curr. Biol. 16, R424–R433 (2006).
Friml, J. Auxin transport—shaping the plant. Curr. Opin. Plant Biol. 6, 7–12 (2003).
Chen, R.J. et al. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc. Natl. Acad. Sci. USA 95, 15112–15117 (1998).
Müller, A. et al. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 17, 6903–6911 (1998).
Bennett, M.J. et al. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273, 948–950 (1996).
Marchant, A. et al. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14, 589–597 (2002).
Marchant, A. et al. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 18, 2066–2073 (1999).
Negi, S., Ivanchenko, M.G. & Muday, G.K. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana Plant J. 55, 175–187 (2008).
Geisler, M. & Murphy, A.S. The ABC of auxin transport: the role of p-glycoproteins in plant development. FEBS Lett. 580, 1094–1102 (2006).
Geisler, M. et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 44, 179–194 (2005).
Lewis, D.R., Miller, N.D., Splitt, B.L., Wu, G. & Spalding, E.P. Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis multidrug resistance-like ABC transporter genes. Plant Cell 19, 1838–1850 (2007).
Wu, G., Lewis, D.R. & Spalding, E.P. Mutations in Arabidopsis multidrug resistance-like ABC transporters separate the roles of acropetal and basipetal auxin transport in lateral root development. Plant Cell 19, 1826–1837 (2007).
Terasaka, K. et al. PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 17, 2922–2939 (2005).
Cho, M., Lee, S. & Cho, H.-T. P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell 19, 3930–3943 (2007).
Lin, R. & Wang, H. Two homologous ATP-binding cassette transporter proteins, AtMDR1 and AtPGP1, regulate Arabidopsis photomorphogenesis and root development by mediating polar auxin transport. Plant Physiol. 138, 949–964 (2005).
Murphy, A., Peer, W.A. & Taiz, L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta. 211, 315–324 (2000).
Brown, D.E. et al. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis . Plant Physiol. 126, 524–535 (2001).
Noh, B., Bandyopadhyay, A., Peer, W.A., Spalding, E.P. & Murphy, A.S. Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature. 424, 999–1002 (2003).
Muday, G.K. et al. RCN1-regulated phosphatase activity and EIN2 modulate hypocotyl gravitropism by a mechanism that does not require ethylene signaling. Plant Physiol. 141, 1617–1629 (2006).
Yang, Y., Hammes, U.Z., Taylor, C.G., Schachtman, D.P. & Nielsen, E. High-affinity auxin transport by the AUX1 influx carrier protein. Curr. Biol. 16, 1123–1127 (2006).
Titapiwatanakun, B. et al. ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis . Plant J. 57, 27–44 (2008).
Petrasek, J. et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312, 914–918 (2006).
Blakeslee, J.J. et al. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis . Plant Cell. 19, 131–147 (2007).
Swarup, K. et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10, 946–954 (2008).
Stasinopoulos, T.C. & Hangarter, R.P. Preventing photochemistry in culture media by long-pass light filters alters growth of cultured tissues. Plant Physiol. 93, 1365–1369 (1989).
Ludwig-Muller, J., Sass, S., Sutter, E., Wodner, M. & Epstein, E. Indole-3-butyric acid in Arabidopsis thaliana Plant Growth Reg. 13, 179–187 (1993).
Woodward, A.W. & Bartel, B. Auxin: regulation, action, and interaction. Ann. Bot. (Lond). 95, 707–735 (2005).
Poupart, J., Rashotte, A.M., Muday, G.K. & Waddell, C.S. The rib1 mutant of Arabidopsis has alterations in indole-3-butyric acid transport, hypocotyl elongation, and root architecture. Plant Physiol. 139, 1460–1471 (2005).
Delbarre, A., Muller, P., Imhoff, V. & Guern, J. Comparison of mechanisms controlling uptake and accumulation of 2,3-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta. 198, 532–541 (1996).
Yamamoto, M. & Yamamoto, K.T. Differential effects of 1-naphthaleneacetic acid, indole-3-acetic acid and 2,4-dichlorophenoxyacetic acid on the gravitropic response of roots in an auxin-resistant mutant of Arabidopsis, aux1 . Plant Cell Physiol. 39, 660–664 (1998).
Parry, G. et al. Novel auxin transport inhibitors phenocopy the auxin influx carrier mutation aux1 . Plant J. 25, 399–406 (2001).
Nadella, V., Shipp, M.J., Muday, G.K. & Wyatt, S.E. Evidence for altered polar and lateral auxin transport in the gravity persistent signal (gps) mutants of Arabidopsis . Plant Cell Environ. 29, 682–690 (2006).
Sabatini, S. et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 99, 463–472 (1999).
Ottenschläger, I. et al. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc. Natl. Acad. Sci. USA 100, 2987–2991 (2003).
Buer, C.S. & Muday, G.K. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16, 1191–1205 (2004).
Peer, W.A. et al. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana . Plant Cell 16, 1898–1911 (2004).
Peer, W.A. & Murphy, A.S. Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci. 12, 556–563 (2007).
Swarup, R., Parry, G., Graham, N., Allen, T. & Bennett, M. Auxin cross-talk: integration of signalling pathways to control plant development. Plant Mol Biol. 49, 411–426 (2002).
Jensen, P.J., Hangarter, R.P. & Estelle, M. Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis . Plant Physiol. 116, 455–462 (1998).
Laxmi, A., Pan, J., Morsy, M. & Chen, R. Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana PLoS ONE 3, e1510 (2008).
Noh, B., Murphy, A.S. & Spalding, E.P. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13, 2441–2454 (2001).
Jain, A. et al. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis . Plant Physiol. 144, 232–247 (2007).
Buer, C.S., Sukumar, P. & Muday, G.K. Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis . Plant Physiol. 140, 1384–1396 (2006).
Buer, C.S., Wasteneys, G.O. & Masle, J. Ethylene modulates root-wave responses in Arabidopsis . Plant Physiol. 132, 1085–1096 (2003).
Muday, G.K. & Rahman, A. In Auxin Transport and the Integration of Gravitropic Growth (eds. Gilroy, S. & Masson, P.) 47–78 (Blackwell Publishing, Oxford, 2007).
Thimann, K.V. A History of the Knowledge of Auxin. In Physiology and Biochemistry of Auxins in Plants (eds. Kutacke, M., Bandurski, R. S. & Krekule, J.) 3–20 (SPB Academic Publishing, Prague, 1988).
Reed, R.C., Brady, S.R. & Muday, G.K. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis . Plant Physiol. 118, 1369–1378 (1998).
Vieten, A. et al. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development. 132, 4521–4531 (2005).
Paciorek, T. et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435, 1251–1256 (2005).
Sieburth, L.E. et al. SCARFACE encodes an ARF-GAP that is required for normal auxin efflux and vein patterning in Arabidopsis . Plant Cell 18, 1396–1411 (2006).
Butler, J.H., Hu, S., Brady, S.R., Dixon, M.W. & Muday, G.K. In vitro and in vivo evidence for actin association of the naphthylphthalamic acid-binding protein from zucchini hypocotyls. Plant J. 13, 291–301 (1998).
Imhoff, V., Muller, P., Guern, J. & Delbarre, A. Inhibitors of the carrier-mediated influx of auxin in suspension-cultured tobacco cells. Planta 210, 580–588 (2000).
McCourt, P. & Keith, K. Sterile techniques in Arabidopsis . Methods Mol. Biol. 82, 13–17 (1998).
Buer, C.S., Masle, J. & Wasteneys, G.O. Growth conditions modulate root-wave phenotypes in Arabidopsis . Plant Cell Physiol. 41, 1164–1170 (2000).
Acknowledgements
We appreciate the capture of the images used to illustrate these protocols as provided by Kevin Cooper with the assistance of Poornima Sukumar and Anita McCauley. We appreciate the helpful comments on the text of this procedure from Poornima Sukumar, Sangeeta Negi and Cassie Mattox. We acknowledge the important role of Aaron Rashotte, Shari Brady and Charles Buer in the initial development of these Arabidopsis methods during their tenure in the Muday research laboratory. This work was supported by the USDA Cooperative State Research, Education and Extension Service (grant 2006-35304-17311 to G.K.M.) and microscopic images were possible by purchase of a Leica stereomicroscope with the support of the National Science Foundation Major Research Instrumentation grant (#05-00702).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lewis, D., Muday, G. Measurement of auxin transport in Arabidopsis thaliana. Nat Protoc 4, 437–451 (2009). https://doi.org/10.1038/nprot.2009.1
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.1
This article is cited by
-
Increased branching independent of strigolactone in cytokinin oxidase 2-overexpressing tomato is mediated by reduced auxin transport
Molecular Horticulture (2022)
-
WAVY GROWTH Arabidopsis E3 ubiquitin ligases affect apical PIN sorting decisions
Nature Communications (2022)
-
The lipid code-dependent phosphoswitch PDK1–D6PK activates PIN-mediated auxin efflux in Arabidopsis
Nature Plants (2020)
-
Pin1At regulates PIN1 polar localization and root gravitropism
Nature Communications (2016)
-
Alteration of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long-term effects on root and shoot architecture
Planta (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.