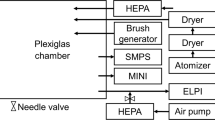
Abstract
This protocol describes the use of solid-phase cytometry for the enumeration of airborne bacteria and fungi. In contrast with conventional methods, accurate results can be obtained in real time, especially for air samples with low numbers of microorganisms. Air samples are collected by impaction on a water-soluble polymer that is subsequently dissolved. Part of the sample can be filtered over two membrane filters with different pore sizes. One filter is used to obtain a total count of all viable microorganisms, and a second filter is used to determine the number of airborne fungi. Microorganisms present on the filter are labeled with a viability substrate and subsequently detected and quantified using a solid-phase cytometer. The detected spots are microscopically validated using an epifluorescence microscope to discriminate between bacteria, fungi and fluorescent particles. The whole procedure takes 5 h to complete and results in the accurate quantification of airborne bacteria and fungi for samples with a low or high microbial load.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lisle, J.T., Hamilton, M.A., Willse, A.R. & McFeters, G.A. Comparison of fluorescence microscopy and solid-phase cytometry methods for counting bacteria in water. Appl. Environ. Microbiol. 70, 5343–6348 (2004).
Mignon-Godefroy, K., Guillet, J.-G. & Butor, C. Solid phase cytometry for detection of rare events. Cytometry 27, 336–344 (1997).
Joux, F. & Lebaron, P. Use of fluorescent probes to assess physiological functions of bacteria at single-cell level. Microbes Infect. 2, 1523–1535 (2000).
Broadaway, S.C., Barton, S.A. & Pyle, B.H. Rapid staining and enumeration of small numbers of total bacteria in water by solid-phase laser cytometry. Appl. Environ. Microbiol. 69, 4272–4273 (2003).
Parthuisot, N., Catala, P., Lemarchand, K., Baudart, J. & Lebaron, P. Evaluation of Chemchrome V6 for bacterial viability assessment in waters. J. Appl. Microbiol. 89, 370–380 (2000).
Reynolds, D.T. & Fricker, C.R. Application of laser scanning for the rapid and automated detection of bacteria in water samples. J. Appl. Microbiol. 86, 785–795 (1999).
De Vos, M.M. & Nelis, H.J. An improved method for the selective detection of fungi in hospital waters by solid phase cytometry. J. Microbiol. Meth. 67, 557–565 (2006).
Van Poucke, S.O. & Nelis, H.J. A 210-min solid phase cytometry test for the enumeration of Escherichia coli in drinking water. J. Appl. Microbiol. 89, 390–396 (2000).
Pyle, B.H., Broadaway, S.C. & McFeters, G.A. Sensitive detection of Escherichia coli O157:H7 in food and water by immunomagnetic separation and solid-phase laser cytometry. Appl. Environ. Microbiol. 65, 1966–1972 (1999).
de Roubin, M.-R. et al. Application of laser scanning cytometry followed by epifluorescent and differental interference contrast microscopy for the detection and enumeration of Cryptoporidium and Giardia in raw and potable waters. J. Appl. Microbiol. 93, 599–607 (2002).
Rushton, P., Place, B.M. & Lightfoot, N.F. An evaluation of a laser scanning device for the detection of Cryptosporidium parvum in treated water samples. Lett. Appl. Microbiol. 30, 303–307 (2000).
Aurell, H. et al. Rapid detection and enumeration of Legionella pneumophila in hot water systems by solid-phase cytometry. Appl. Environ. Microbiol. 70, 1651–1657 (2004).
Pougnard, C. et al. Rapid detection and enumeration of Naegleria fowleri in surface waters by solid-phase cytometry. Appl. Environ. Microbiol. 68, 3102–3107 (2002).
West, N.J. et al. Rapid quantification of the toxic alga Prymnesium parvum in natural samples by use of a specific monoclonal antibody and solid-phase cytometry. Appl. Environ. Microbiol. 72, 860–868 (2006).
Baudart, J., Olaizola, A., Coallier, J., Gauthier, V. & Laurent, P. Assessment of a new technique combining a viability test, whole-cell hybridization and laser-scanning cytometry for the direct counting of viable Enterobacteriaceae cells in drinking water. FEMS Microbiol. Lett. 243, 405–409 (2005).
Lepeuple, S., Delabre, K., Gilouppe, S., Intertaglia, L. & de Roubin, M.R. Laser scanning detection of FISH-labelled Escherichia coli from water samples. Water Sci. Technol. 47, 123–129 (2003).
Töbe, K., Eller, G. & Medlin, L.K. Automated detection and enumeration for toxic algae by solid-phase cytometry and the introduction of a new probe for Prymnesium parvum (Haptophyta: Prymnesiophyceae). J. Plankt. Res. 28, 643–657 (2006).
Cools, I. et al. Solid phase cytometry as a tool to detect viable but non-culturable cells of Campylobacter jejuni . J. Microbiol. Meth. 63, 107–114 (2005).
Vanhee, L.M.E., Nelis, H.J. & Coenye, T. Enumeration of airborne bacteria and fungi using solid phase cytometry. J. Microbiol. Meth. 72, 12–19 (2008).
De Vos, M.M. & Nelis, H.J. Detection of Aspergillus fumigatus hyphae by solid phase cytometry. J. Microbiol. Meth. 55, 557–564 (2003).
De Vos, M.M., Sanders, N.N. & Nelis, H.J. Detection of Aspergillus fumigatus hyphae in respiratory secretions by membrane filtration, fluorescent labeling and laser scanning. J. Microbiol. Meth. 64, 420–423 (2006).
Bauters, T.G.M., Swinne, D., Stove, V. & Nelis, H.J. Detection of single cells of Cryptococcus neoformans in clinical samples by solid-phase cytometry. J. Clin. Microbiol. 41, 1736–1737 (2003).
Catala, P. et al. Effectiveness of CSE to counterstain particles and dead bacterial cells with permeabilised membranes: application to viability assessment in waters. FEMS Microbiol. Lett. 178, 219–226 (1999).
Cruz, P. & Buttner, M.P. Analysis of bioaerosol samples. In Manual of Environmental Microbiology 3rd edn. (eds. Hurst, C.J. et al.) 68.952–68.960 (ASM Press, Washington D.C., USA, 2007).
Lemarchand, K., Parthuisot, N., Catala, P. & Lebaron, P. Comparative assessment of epifluorescence microscopy, flow cytometry and solid-phase cytometry used in the enumeration of specific bacteria in water. Aquat. Microbial. Ecol. 25, 301–309 (2001).
Prigione, V., Lingua, G. & Filipello Marchisio, V. Development and use of flow cytometry for detection of airborne fungi. Appl. Environ. Microbiol. 70, 1360–1365 (2004).
Alvarez, A.J., Buttner, M.P. & Stetzenbach, L.D. PCR for bioaerosol monitoring: sensitivity and environmental interference. Appl. Environ. Microbiol. 61, 3639–3644 (1995).
Giovannangelo, M.E.C.A. et al. Levels and determinants of β (1 → 3)-glucans and fungal extracellular polysaccharides in house dust of (pre-)schoolchildren in three European countries. Environ. Internat. 33, 9–16 (2007).
Robine, E., Lacaze, I., Moularat, S., Ritoux, S. & Boissier, M. Characterisation of exposure to airborne fungi: measurement of ergosterol. J. Microbiol. Meth. 63, 185–192 (2005).
Venkateswaran, K., Hattori, N., La Duc, M.T. & Kern, R. ATP as a biomarker of viable microorganisms in clean-room facilities. J. Microbiol. Meth. 52, 367–377 (2003).
Acknowledgements
We acknowledge the excellent technical assistance of M. Battista. This research was financially supported by the Bijzonder Onderzoeksfonds of Ghent University (project B/07601/02).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Vanhee, L., Nelis, H. & Coenye, T. Detection and quantification of viable airborne bacteria and fungi using solid-phase cytometry. Nat Protoc 4, 224–231 (2009). https://doi.org/10.1038/nprot.2008.228
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.228
This article is cited by
-
Linking the conventional and emerging detection techniques for ambient bioaerosols: a review
Reviews in Environmental Science and Bio/Technology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.