Abstract
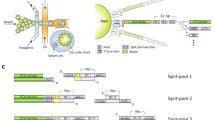
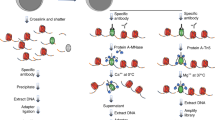
The bead transfection method involves binding nucleic acids onto 3-μm-diameter paramagnetic beads, treating the beads with transfection reagent, and using them as scaffolds to direct transfection to individual cells or regions in a population. Typically, PCR products are used because they can be conveniently generated using biotinylated primers and can introduce site-directed mutations, without the need for cloning or plasmid purification. However, the method can be adapted to transfect plasmid DNA or RNA. The magnetic properties of the beads allows magnets to direct the loci of transfection in cell culture; magnetic arrays are built in cell culture chambers to allow multiple parallel transfections on the same microscope coverslip. The PCR reaction and transfection can be carried out in 1 d, and transfection results can be viewed in 24–48 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Ziauddin, J. & Sabatini, D.M. Microarrays of cells expressing defined cDNAs. Nature 411, 107–110 (2001).
Palmer, E. & Freeman, T. Cell-based microarrays: current progress, future prospects. Pharmacogenomics 6, 527–534 (2005).
Erfle, H., Simpson, J.C., Bastiaens, P.I. & Pepperkok, R. siRNA cell arrays for high-content screening microscopy. Biotechniques 37, 454–458, 460, 462 (2004).
Wheeler, D.B. et al. RNAi living-cell microarrays for loss-of-function screens in Drosophila melanogaster cells. Nat. Methods 1, 127–132 (2004).
Isalan, M., Santori, M.I., Gonzalez, C. & Serrano, L. Localized transfection on arrays of magnetic beads coated with PCR products. Nat. Methods 2, 113–118 (2005).
Heald, R. et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425 (1996).
Isalan, M. Construction of semi-randomized gene libraries with weighted oligonucleotide synthesis and PCR. Nat. Protocols 1, (doi 10.1038/nprot.2006.68) (2006).
Isalan, M., Lemerle, C. & Serrano, L. Engineering gene networks to emulate Drosophila embryonic pattern formation. PLoS Biol. 3, e64 (2005).
Kamau, S.W. et al. Enhancement of the efficiency of non-viral gene delivery by application of pulsed magnetic field. Nucleic Acids Res. 34, e40 (2006).
Cormack, B.P., Valdivia, R.H. & Falkow, S. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173, 33–38 (1996).
Osborn, M. & Weber, K. Immunofluorescence and immunocytochemical procedures with affinity purified antibodies: tubulin-containing structures. Methods Cell Biol. 24, 97–132 (1982).
Chen, F., MacDonald, C.C. & Wilusz, J. Cleavage site determinants in the mammalian polyadenylation signal. Nucleic Acids Res. 23, 2614–2620 (1995).
Innis, M.A. PCR Protocols: A Guide to Methods and Applications (Academic Press, San Diego, 1990).
Scherer, F. et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 9, 102–109 (2002).
Acknowledgements
M.I. was supported by an International Research Fellowship from the Wellcome Trust, UK. M.I.S was funded by a Fundacion Carolina Postdoctoral Fellowship. We would like to thank G. DeCarcer Diez and K. Michalodimitrakis for help in the design of the transfection chamber and D. Megias Vazquez for assistance with confocal imaging.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Santori, M., Gonzalez, C., Serrano, L. et al. Localized transfection with magnetic beads coated with PCR products and other nucleic acids. Nat Protoc 1, 526–531 (2006). https://doi.org/10.1038/nprot.2006.74
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.74
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.