Abstract
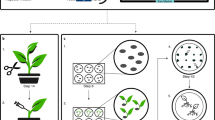
This protocol is used to produce stably transformed tobacco (Nicotiana tabacum) NT1 cell lines, using Agrobacterium tumefaciens–mediated DNA delivery of a binary vector containing a gene encoding hepatitis B surface antigen and a gene encoding the kanamycin selection marker. The NT1 cultures, at the appropriate stage of growth, are inoculated with A. tumefaciens containing the binary vector. A 3-day cocultivation period follows, after which the cultures are rinsed and placed on solid selective medium. Transformed colonies ('calli') appear in approximately 4 weeks; they are subcultured until adequate material is obtained for analysis of antigen production. 'Elite' lines are selected based on antigen expression and growth characteristics. The time required for the procedure from preparation of the plant cell materials to callus development is approximately 5 weeks. Growth of selected calli to sufficient quantities for antigen screening may require 4–6 weeks beyond the initial selection. Creation of the plasmid constructs, transformation of the A. tumefaciens line, and ELISA and Bradford assays to assess protein production require additional time.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Nagata, T., Nemoto, Y. & Hasezawa, S. Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int. Rev. Cytol. 132, 1–30 (1992).
Matsumoto, S., Ikura, K., Ueda, M. & Sasaki, R. Characterization of a human glycoprotein (erythropoietin) produced in cultured tobacco cells. Plant Mol. Biol. 27, 1163–1172 (1995).
Sunil Kumar, G.B., Ganapathi, T.R., Revanthi, C.J., Prasad, K.S.N. & Bapat, V.A. Expression of hepatitis B surface antigen in tobacco cell suspension cultures. Protein Expr. Purif. 32, 10–17 (2003).
Sojikul, P., Buehner, N. & Mason, H.S. A plant signal peptide–hepatitis B surface antigen fusion protein with enhanced stability and immunogenicity expressed in plant cells. Proc. Natl. Acad. Sci. USA 100, 2209–2214 (2003).
Judge, N.A., Mason, H.S. & O'Brien, A.D. Plant cell-based intimin vaccine given orally to mice primed with intimin reduces time of Escherichia coli O157:H7 shedding in feces. Infect. Immun. 72, 168–175 (2004).
Xu, J., Shpak, E., Gu, T., Moo-Young, M. & Kieliszewski, M. Production of recombinant plant gum with tobacco cell culture in bioreactor and gum characterization. Biotechnol. Bioeng. 90, 578–588 (2005).
Zhang, X. & Mason, H.S. Bean yellow dwarf virus replicons for high-level transgene expression in transgenic plants and cell cultures. Biotechnol. Bioeng. 93, 271–279 (2006).
Newman, T.C., Ohme-Takagi, M., Taylor, C.B. & Green, P.J. DST sequences, highly conserved among plant SAUR genes, target reporter transcripts for rapid decay in tobacco. Plant Cell 5, 701–714 (1993).
Tacket, C.O. et al. Immunogenicity in humans of a recombinant bacterial antigen delivered in transgenic potato. Nat. Med. 4, 607–609 (1998).
Kong, Q. et al. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc. Natl. Acad. Sci. USA 98, 11539–11544 (2001).
Zor, T. & Selinger, Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal. Biochem. 236, 302–308 (1996).
Acknowledgements
The authors thank L. Richter, M. Smith and Z. Huang for development and optimization of the ELISA protocol. This work was supported by grants R01 AI042836 from the National Institutes of Health and BES0109936 from National Science Foundation to H.S.M.
Author information
Authors and Affiliations
Contributions
B.J.G., preparation of screening procedure and sections related to screening and analysis. K.J.M., preparation of plant transformation procedure, preliminary text and photos. H.S.M., editing of text, editorial communications and advisor.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mayo, K., Gonzales, B. & Mason, H. Genetic transformation of tobacco NT1 cells with Agrobacterium tumefaciens. Nat Protoc 1, 1105–1111 (2006). https://doi.org/10.1038/nprot.2006.176
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.176
This article is cited by
-
Functional MYB transcription factor gene HtMYB2 is associated with anthocyanin biosynthesis in Helianthus tuberosus L
BMC Plant Biology (2020)
-
Generation of transgenic cell suspension cultures of the model legume Medicago truncatula: a rapid method for Agrobacterium mediated gene transfer
Plant Cell, Tissue and Organ Culture (PCTOC) (2019)
-
Dramatic secretion of recombinant protein expressed in tobacco cells with a designer glycopeptide tag is highly impacted by medium composition
Plant Cell Reports (2016)
-
A Dual-Intein Autoprocessing Domain that Directs Synchronized Protein Co-Expression in Both Prokaryotes and Eukaryotes
Scientific Reports (2015)
-
Expression of the acidic-subunit of amarantin, carrying the antihypertensive biopeptides VY, in cell suspension cultures of Nicotiana tabacum NT1
Plant Cell, Tissue and Organ Culture (PCTOC) (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.