Abstract
Cannabis use increases rates of psychotic relapse and treatment failure in schizophrenia patients. Clinical studies suggest that cannabis use reduces the efficacy of antipsychotic drugs, but there has been no direct demonstration of this in a controlled study. The present study demonstrates that exposure to the principal phytocannabinoid, Δ9-tetrahydrocannabinol (THC), reverses the neurobehavioral effects of the antipsychotic drug risperidone in mice. THC exposure did not influence D2 and 5-HT2A receptor binding, the major targets of antipsychotic action, but it lowered the brain concentrations of risperidone and its active metabolite, 9-hydroxy risperidone. As risperidone and its active metabolite are excellent substrates of the ABC transporter P-glycoprotein (P-gp), we hypothesized that THC might increase P-gp expression at the blood–brain barrier (BBB) and thus enhance efflux of risperidone and its metabolite from brain tissue. We confirmed that the brain disposition of risperidone and 9-hydroxy risperidone is strongly influenced by P-gp, as P-gp knockout mice displayed greater brain concentrations of these drugs than wild-type mice. Furthermore, we demonstrated that THC exposure increased P-gp expression in various brain regions important to risperidone’s antipsychotic action. We then showed that THC exposure did not influence the neurobehavioral effects of clozapine. Clozapine shares a very similar antipsychotic mode of action to risperidone, but unlike risperidone is not a P-gp substrate. Our results imply that clozapine or non-P-gp substrate antipsychotic drugs may be better first-line treatments for schizophrenia patients with a history of cannabis use.
Similar content being viewed by others
Introduction
Cannabis use increases rates of psychotic relapse and treatment failure in schizophrenia patients. This is a major problem as 40% of schizophrenia patients have a cannabis use history (Koskinen et al, 2010; Manrique-Garcia et al, 2014). A study of over 2000 first-episode psychosis (FEP) patients suggested that the poor clinical outcomes observed in cannabis-using patients might be explained by cannabis decreasing antipsychotic efficacy—patients with a cannabis use history were prescribed a greater number of unique antipsychotic drugs, a proxy measure of clinical judgment of antipsychotic drug failure (Patel et al, 2016). However, there is no direct evidence that cannabinoids decrease the pharmacological actions of antipsychotic drugs. Here we provide data in mice showing cannabinoid exposure reduces antipsychotic efficacy.
Animal studies provide greater experimental control to investigate interactions between cannabinoids and antipsychotic drugs. Moreover, they provide an efficient means to delineate biological mechanisms that may inform novel treatment strategies. A number of mechanisms may play a role in the interaction between cannabis and antipsychotic drugs. Pharmacodynamically, cannabinoids may reduce antipsychotic efficacy by affecting major antipsychotic drug targets such as dopamine D2 and serotonin 5-HT2A receptors. Alternatively, pharmacokinetic mechanisms may be involved, where phytocannabinoids could induce cytochrome P450 enzymes involved in antipsychotic drug metabolism. An emerging pharmacokinetic mechanism for clinically significant drug interactions involves ATP-binding cassette (ABC) transporters (Montanari and Ecker, 2015). P-glycoprotein (P-gp) is a well-characterized ABC transporter that is localized at the blood–brain barrier (BBB) and strongly limits the brain accumulation of CNS drugs including various antipsychotics. P-gp at the BBB binds antipsychotic drugs such as risperidone, paliperidone (9-hydroxy risperidone), olanzapine, quetiapine, amisulpride, and aripiprazole, and transports them from brain tissue back into the blood (Boulton et al, 2002; Doran et al, 2005; Linnet and Ejsing, 2008; Moons et al, 2011). Interestingly clozapine, the drug of choice for drug-resistant patients, has poor affinity for P-gp (Boulton et al, 2002; Doran et al, 2005). The mechanisms underlying clozapine’s superior efficacy have not been clearly resolved despite 30 years of research (Joober and Boksa, 2010; Remington et al, 2016). We hypothesize that clozapine’s lack of affinity for P-gp contributes to its favorable therapeutic properties in drug-resistant patients and those with a cannabis use history.
In the present study we demonstrate that exposure to the main psychoactive constituent of cannabis, Δ9-tetrahydrocannabinol (THC), reduces the neurobehavioral efficacy of risperidone but not clozapine in mice. Furthermore, we delineate the biological mechanism subserving the interaction between THC and risperidone that involves P-gp at the BBB.
Materials and methods
Mice
A total of 210 male C57BL/6 mice aged 9 weeks were used for this study, along with 8 male wild-type (WT) and 8 P-gp knockout (KO) mice aged between 16 and 20 weeks (Taconic Farms, USA) (see Brzozowska et al, 2016; Spiro et al, 2012). Animals were housed in standard mouse cages, under a 12 h light/dark schedule, with food and water available ad libitum. The University of Sydney’s Animal Ethics Committee approved all experimental procedures undertaken (Protocol number: K21/1-2013/3/5924).
Drugs
THC (THC Pharm, Germany), risperidone, and clozapine (Sequoia, UK) were administered to mice as previously described, see (Boucher et al, 2007, 2011; Brzozowska et al, 2016; Todd and Arnold, 2016). Based on the FDA species interconversion formula a dose of 1 mg/kg of THC in mice is equivalent to 5 mg of THC in humans (Food and Drug Administration, 2005; Reagan-Shaw et al, 2008). A 1 mg/kg i.p. dose of THC has discriminative stimulus effects in mice (Vann et al, 2008) and 5 mg of THC produces subjective high in human cannabis users (Hindocha et al, 2015). The doses of risperidone (0.3–1 mg/kg) and clozapine (3 mg/kg) selected are equivalent to the doses used clinically at the commencement of antipsychotic drug treatment, with human dose equivalents of 1.5–5 mg of risperidone and 15 mg of clozapine (Food and Drug Administration, 2005).
Experimental Design
Mice were divided equally into three groups: two groups were injected with vehicle (VEH) and the third group with THC (1 mg/kg) for 14 days. Fourteen days after the final pretreatment injection, mice were challenged with either VEH or an antipsychotic drug (risperidone or clozapine) yielding the following groups: VEH-VEH, VEH-antipsychotic drug, and THC-antipsychotic drug. A washout period of 14 days was applied to ensure the results obtained were enduring effects of THC, and not due to residual THC actions, as THC is stored in adipose tissue for long periods of time (Gunasekaran et al, 2009; Kreuz and Axelrod, 1973). This experimental design was used to assess c-Fos immunohistochemistry, prepulse inhibition of startle (PPI), and locomotor activity (see Supplementary Information for detailed methods). Separate cohorts of mice were treated with VEH or THC as described above before sample collection for D2 and 5-HT2A receptor autoradiography, risperidone and THC tissue concentrations, and P-gp immunofluorescence (see Supplementary Information for details).
Statistical Analysis
Data for risperidone c-Fos immunoreactivity and P-gp immunofluorescence were analyzed using the nonparametric Mann–Whitney U-test, as the data violated the homogeneity of variance assumption of ANOVA and could not be corrected. The risperidone and clozapine PPI and locomotor activity data were analyzed using one-way ANOVA with group as the between subject factor followed by Student–Newman–Keuls post hoc tests. Autoradiography data were analyzed using two-way ANOVA with drug treatment and brain region as the between subject factors. Region-specific binding affinities were analyzed using Student’s unpaired t-test. Risperidone and 9-hydroxy risperidone brain and plasma concentrations were analyzed using two-way ANOVA with between subject factors of THC treatment and risperidone dose or genotype and time. Differences between groups were deemed statistically significant when P<0.05.
Results
Repeated THC Exposure Reduced the Acute Neurobehavioral Effects of Risperidone
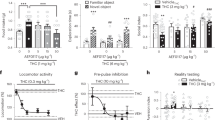
Mice were treated with THC daily for 14 days before a 14-day washout period. They were then challenged with risperidone and brain activation was assessed using c-Fos immunohistochemistry. Risperidone significantly increased c-Fos expression in various brain regions (Figure 1 and Supplementary Table 1). Repeated preexposure to THC abolished risperidone-induced c-Fos expression in the ventrolateral septum (Figure 1a), the medial and dorsomedial caudate putamen (Figure 1b), the nucleus accumbens shell (Figure 1c), and the paraventricular nucleus of the thalamus (Figure 1d).
Repeated THC exposure reduced the neurobehavioral effects of the antipsychotic drug risperidone. c-Fos expression in the (a) ventrolateral septum (LSV), (b) dorsomedial caudate putamen (CPu), (c) nucleus accumbens shell (AcbSh), and (d) paraventricular nucleus of the thalamus (PV) (n=8 per group). (e) %PPI, (f) startle response, and (g) locomotor activity (n=11–12 per group). VEH, vehicle; THC, 1 mg/kg Δ9-tetrahydrocannabinol; RISP0.3, 0.3 mg/kg risperidone; RISP1, 1 mg/kg risperidone. Data presented as mean+SEM. Significant differences compared with VEH-VEH control *P<0.05 and **P<0.01, and between VEH-RISP and THC-RISP groups #P<0.05 (Mann–Whitney U for c-Fos or SNK post hoc test for behavior). For PPI, startle response, and locomotor activity, one-way ANOVA revealed an overall group effect (F(4, 53)=3.1, P<0.05; F(4, 53)=4.3, P<0.01; F(4, 53)=7.3, P<0.0001, respectively).
We then assessed whether the reversal of risperidone-induced brain activation by THC had implications for the behavioral effects of risperidone. To do this we examined locomotor activity and sensorimotor gating, two measures sensitive to the effects of antipsychotic drugs in mice and humans (Csomor et al, 2014; Ouagazzal et al, 2001; Quednow et al, 2006). Using the same THC treatment regimen mice were again challenged with risperidone, and PPI and locomotor activity was assessed. Vehicle pretreated mice challenged with 1 mg/kg risperidone but not 0.3 mg/kg risperidone displayed significantly increased %PPI (Figure 1e). THC pretreatment abolished risperidone-induced PPI facilitation, as the THC pretreated mice challenged with risperidone (THC-RISP1) were no different to the vehicle-vehicle control mice (VEH-VEH) and had significantly less %PPI than the vehicle pretreated mice challenged with risperidone (VEH-RISP1). During PPI testing, we also measured the startle response of the animals to a 120 dB acoustic stimulus (Figure 1f). Challenge with 1 mg/kg but not 0.3 mg/kg risperidone significantly decreased the startle response compared with the vehicle control. However, THC pretreatment did not affect the impaired startle response in mice treated with 1 mg/kg risperidone. This highlights that the effects of THC on risperidone-induced sensorimotor gating and startle response can be dissociated. These results provide yet another example of how startle reactivity and PPI can be dissociated in pharmacological studies, where drugs affect higher-order neural circuitry to influence PPI, with startle reactivity being affected by drug actions on brainstem circuits (Geyer et al, 2001; Li et al, 2009).
The results for locomotor activity over 60 min for risperidone-challenged mice are shown in Figure 1g. Challenge injection with both 0.3 and 1 mg/kg risperidone significantly decreased locomotor activity compared with vehicle controls. This decrease in locomotor activity was attenuated by THC pretreatment in mice challenged with 0.3 mg/kg, as the THC-RISP0.3 group had significantly higher locomotor activity than the VEH-RISP0.3 group. However, THC did not blunt the locomotor suppressant effects of 1 mg/kg risperidone. Taken together, these results demonstrate that repeated treatment with THC reduces the acute neurobehavioral effects of the commonly used antipsychotic drug risperidone. These results cannot be explained by functional antagonism as a further control experiment indicated that THC pretreatment alone did not significantly reduce PPI or increase locomotor activity following a 2-week washout period (see Supplementary Table 2). In addition, blood samples collected after the THC washout period showed no detectable levels of THC or its terminal metabolite THC-COOH (see Supplementary Table 2).
Repeated THC Exposure Did Not Affect Brain D2 or 5-HT2A Receptor Binding
We then sought to determine whether THC reduced the efficacy of risperidone due to a pharmacodynamic mechanism via reduced D2 and 5-HT2A receptor binding, the two main antipsychotic drug targets of risperidone. The mean specific D2 ([3H] raclopride) and 5-HT2A ([3H] ketanserin) receptor binding density of vehicle and THC pretreated groups in various brain regions is shown in Figure 2. THC exposure did not alter D2 or 5-HT2A receptor binding density in any of the brain regions examined, with both THC and vehicle pretreated groups obtaining similar binding concentrations (Figure 2a and b).
THC exposure did not influence binding or expression of D2 and 5-HT2A receptors, the major targets of antipsychotic drug action. (a) [3H] raclopride (D2 antagonist) binding and representative autoradiograph. (b) [3H] ketanserin (5-HT2A antagonist) binding and representative autoradiograph (n=6 per group). LSV, ventrolateral septum; CPu, dorsomedial caudate putamen; AcbSh, nucleus accumbens shell; AcbC, nucleus accumbens core; PrL, prelimbic cortex; VEH, vehicle; THC, Δ9-tetrahydrocannabinol. Data represent mean+SEM. No main effects of THC in two-way ANOVA (THC by brain region) Ps>0.05. This was confirmed with individual unpaired t-tests for each brain region that indicated no significant differences between vehicle and THC pretreated groups for either D2 or 5-HT2A receptor binding.
Repeated THC Exposure Reduced the Brain Concentrations of Risperidone and 9-Hydroxy Risperidone
As THC exposure did not affect D2 or 5-HT2A receptor binding, we then explored whether THC treatment impaired the brain disposition of risperidone. This would provide an alternative explanation for the ability of THC to reduce the neurobehavioral effects of risperidone. We therefore examined whether THC exposure altered the brain concentration of risperidone and its active metabolite 9-hydroxy risperidone. THC-treated mice had significantly decreased brain concentrations compared with vehicle controls of risperidone (Figure 3a), 9-hydroxy risperidone (Figure 3b), and the total active drug moiety (the combined brain concentration of risperidone and 9-hydroxy risperidone) (Figure 3c), following risperidone challenge. There was clearly a dose-dependent effect of risperidone challenge on the brain concentrations of risperidone, 9-hydroxy risperidone, and total active moiety, but there were no significant interactions between THC and risperidone dose on the brain concentrations in the two-way ANOVA.
Repeated THC exposure reduced whole brain concentrations of risperidone, its active metabolite 9-hydroxy risperidone, and total active moiety. (a) Risperidone, (b) 9-hydroxy risperidone, and (c) total active moiety brain concentrations (n=6–8 per group). VEH, vehicle; THC, Δ9-tetrahydrocannabinol; RISP0.3, 0.3 mg/kg risperidone; RISP1, 1 mg/kg risperidone. Data represent mean+SEM. Significant differences denoted reflect a main effect of THC in two-way ANOVA **P<0.01, ***P<0.001 and ****P<0.0001. Two-way ANOVA indicated that THC-treated mice had significantly decreased brain concentrations compared with vehicle of risperidone (F(1, 26)=10, P<0.01), 9-hydroxy risperidone (F(1, 26)=19.1, P<0.001), and the total active drug moiety (F(1, 26)=17, P<0.0001). There was a significant effect of risperidone dose on the brain concentrations of risperidone (F(1, 26)=23.2, P<0.0001), 9-hydroxy risperidone (F(1, 26)=18.6, P<0.0001), and total active moiety (F(1, 26)=29.3, P<0.0001), but there were no significant interactions between THC and risperidone dose on these brain concentrations.
THC Exposure May Reduce Brain Risperidone Concentrations by Inducing P-gp Expression at the BBB
Alteration in the metabolism of risperidone cannot explain the current findings, as THC decreased the brain concentrations of both risperidone and the 9-hydroxy metabolite. Moreover, this conclusion is consistent with risperidone’s major cytochrome P450 (CYP450) metabolizing enzymes not being inducible by xenobiotics, at least based on the currently available evidence (ie, CYP2D6 in humans and CYP2D9–13, 22, 26, 34, and 40 in mice) (Miksys et al, 2005; Murray, 2006; Nelson et al, 2004; Sheehan et al, 2010; Urichuk et al, 2008). Our results therefore suggest that THC exposure impaired the neurobehavioral actions of risperidone, not by altering receptor binding or drug metabolism, but by interfering with risperidone disposition leading to subtherapeutic brain concentrations. Considering these findings, we hypothesized that the THC-induced reduction in brain risperidone levels might be explained by enhanced expression of the ABC transporter P-gp. Such a theory is supported by the observation that P-gp strongly influences the brain disposition of various antipsychotic drugs including risperidone and its active 9-hydroxy metabolite (Boulton et al, 2002; Doran et al, 2005). To confirm this, we measured the concentrations of risperidone and 9-hydroxy risperidone in P-gp KO mice to prove P-gp regulates the brain disposition of these drugs. P-gp KO mice retained significantly more risperidone and 9-hydroxy risperidone in the brain and plasma at 1 and 3 h after administration (Figure 4a–d). Overall, our results are consistent with prior research showing P-gp at the BBB plays a dominant role in regulating the brain disposition of risperidone and 9-hydroxy risperidone, with the brain/plasma concentration ratios being markedly elevated in P-gp KO compared with WT mice (Boulton et al, 2002; Brzozowska et al, 2016; Doran et al, 2005; Wang et al, 2004).
The brain disposition of risperidone and 9-hydroxy risperidone is regulated by the ABC transporter P-gp and repeated THC exposure increased brain P-gp expression. Brain (a) risperidone and (b) 9-hydroxy risperidone concentrations, as well as plasma (c) risperidone and (d) 9-hydroxy risperidone concentrations in WT and P-gp KO mice (n=5–8 per group). (e) Representative images of P-gp immunofluorescence in mouse brain and (f) P-gp expression in different brain regions of mice treated with VEH or THC. Data represent mean+SEM. WT; wild type; P-gp, P-gp KO mice; VEH, vehicle; THC, Δ9-tetrahydrocannabinol; LSV, ventrolateral septum; CPu, dorsomedial caudate putamen; AcbC, nucleus accumbens core; PV, paraventricular nucleus of the thalamus. Significant differences in KO mice studies reflect a main effect of P-gp genotype in two-way ANOVA, *P<0.05, **P<0.01 and ****P<0.0001. Specifically, we found a main effect of P-gp genotype for both brain and plasma risperidone concentrations (F(1, 20)=37.6, P<0.0001; F(1, 18)=7.7, P<0.05, respectively) and brain and plasma 9-hydroxy risperidone concentrations (F(1, 20)=31.4, P<0.0001; F(1, 18)=10.8, P<0.01, respectively). There were no effects of time or genotype by time interactions for brain and plasma risperidone concentrations. However, 9-hydroxy risperidone brain and plasma concentrations increased over time (F(1, 20=10.3, P<0.01; F(1, 18)=67.8, P<0.0001, respectively) and did more so in the P-gp KO mice supported by P-gp genotype by time interactions (F(1, 20)=8.9, P<0.01; F(1, 18)=6.3, P<0.05, respectively). Repeated THC exposure significantly increased the cumulative volume of P-gp transporter expression in key brain regions relevant to antipsychotic action (n=4–6 per group). Mann–Whitney U-tests *P<0.05.
The increased brain concentrations of risperidone and 9-hydroxy risperidone found in P-gp KO mice imply that P-gp at the BBB determines the brain accumulation of these drugs. Given these findings we hypothesized that the THC-induced reduction in brain risperidone and active metabolite concentrations might be explained by enhanced P-gp expression at the BBB. To test this we examined the expression of P-gp transporter protein in THC-treated mice using immunofluorescence, where the cumulative volume of P-gp immunofluorescence was assessed within a constant sampling area (Figure 4e and f). THC significantly increased the cumulative volume of P-gp in the ventrolateral septum, nucleus accumbens core, and the paraventricular nucleus of the thalamus compared with vehicle controls (Figure 4f). There was no significant difference in P-gp expression in the dorsomedial caudate putamen, nucleus accumbens shell, prefrontal cortex, and paraventricular hypothalamic nucleus (see Supplementary Table 3).
Repeated THC Exposure Did Not Influence the Acute Neurobehavioral Effects of Clozapine
Taken together, our results suggest that THC exposure reduced the neurobehavioral effects of risperidone because of THC-induced upregulation of P-gp expression at the BBB and the consequent enhanced brain extrusion of the P-gp substrates risperidone and 9-hydroxy risperidone. Our model therefore predicts that THC exposure will not affect the efficacy of antipsychotic drugs that are not ABC transporter substrates. Clozapine is not a P-gp substrate (Boulton et al, 2002; Doran et al, 2005) but shares similar pharmacodynamic mechanisms of action to risperidone and is the drug of choice for treatment-resistant schizophrenia patients. We therefore assessed whether repeated THC exposure affected the acute neurobehavioral effects of clozapine. We first examined the effects of repeated THC exposure on clozapine-induced c-Fos expression. Clozapine exposure increased c-Fos expression in the ventrolateral septum (Figure 5a), prelimbic cortex (Figure 5b), nucleus accumbens shell (Figure 5c), and cingulate cortex (see Supplementary Table 4). However, repeated THC exposure did not influence clozapine-induced c-Fos expression in any of the brains regions examined. Similarly, for behavior, repeated THC exposure did not influence the actions of clozapine on sensorimotor gating or locomotor activity (Figure 5d–f). Clozapine significantly increased %PPI (Figure 5d) and decreased startle response (Figure 5e) and locomotor activity (Figure 5f). However, contrary to the results observed with risperidone, repeated THC exposure did not affect clozapine-induced PPI facilitation and suppression of startle response and locomotor activity.
Repeated THC exposure did not influence the acute neurobehavioral effects of the non-P-gp substrate clozapine. c-Fos expression in the (a) ventrolateral septum, (b) prelimbic cortex, and (c) nucleus accumbens shell (n=6 per group). (d) %PPI, (e) startle response, and (f) locomotor activity (n=12–24 per group). Data represent mean+SEM. VEH, vehicle; THC, Δ9-tetrahydrocannabinol; CLOZ, 3 mg/kg clozapine. Significant difference to VEH-VEH indicated using SNK post hoc *P<0.05. NS, no significant effect comparing VEH-CLOZ with THC-CLOZ. For c-Fos data in the LSV, PrL, and AcbSh, one-way ANOVA revealed an overall group effect (F(2, 12)=9.89, P<0.05; F(2, 12)=9.30, P<0.05; F(2, 12)=20.3, P<0.05, respectively). For %PPI, startle response, and locomotor activity, one-way ANOVA revealed an overall group effect (F(2, 41)=6.7, P<0.01; F(2, 41)=11, P<0.001; F(2, 41)=13.6, P<0.0001, respectively).
Discussion
Cannabis use increases rates of psychotic relapse and treatment failure (Manrique-Garcia et al, 2014). This problem is significant as 40% of schizophrenia patients use cannabis (Koskinen et al, 2010). No controlled studies have addressed whether cannabis use worsens treatment outcomes in schizophrenia patients by decreasing the efficacy of antipsychotic drugs. A recent clinical study showed poor clinical outcomes in cannabis-using patients were associated with an increased number of unique antipsychotic drug prescriptions, a proxy measure of clinical judgment of antipsychotic drug failure (Patel et al, 2016). Here we provide direct evidence in a controlled mouse study that preexposure to the most abundant phytocannabinoid in street cannabis, THC (Swift et al, 2013), reduces the neurobehavioral effects of the widely used antipsychotic drug risperidone. Furthermore, we identified a mechanism that accounted for the capacity of THC to decrease the efficacy of risperidone but not clozapine, a drug effective in treatment-resistant patients (Howes et al, 2012). Our results suggest that the differential binding of antipsychotic drugs to the ABC transporter P-gp may predict cannabis–antipsychotic drug interactions.
Mice exposed to THC displayed profoundly reduced neurobehavioral responses to risperidone. Antipsychotics induce c-Fos expression, a marker of neuronal activation, in various brain regions (Fibiger, 1994; Sumner et al, 2004). THC preexposure reversed risperidone-induced c-Fos expression in the brain of mice challenged 2 weeks after the final THC exposure. The ability of THC preexposure to reverse risperidone-induced brain activation had functional implications, as THC preexposure also significantly reduced the ability of risperidone to facilitate PPI and suppress locomotor activity. These effects were not due to physiological antagonism, as THC pretreatment did not alter PPI or locomotor activity in mice challenged with vehicle 2 weeks after the final THC exposure. Furthermore, these effects could not be explained by residual THC interfering directly with the efficacy of risperidone, as THC was not present in the blood 2 weeks after the final pretreatment dose.
D2 receptor antagonism is necessary for all antipsychotic drugs to reduce positive symptoms of schizophrenia (Kapur and Remington, 2001; Kapur et al, 2000). Furthermore, 5-HT2A receptors are critical to the actions of the hallucinogens LSD and psilocybin and are antagonized by both risperidone and clozapine (Glennon et al, 1984; Nichols, 2004). This prompted the question of whether THC reduced the neurobehavioral effects of risperidone by altering D2 and 5-HT2A receptor binding. However, THC exposure did not affect D2 and 5-HT2A binding in our autoradiography study. As important pharmacodynamic targets of antipsychotics were unaffected by THC exposure, we turned our attention to a pharmacokinetic explanation. Indeed, THC treatment reduced the brain concentrations of risperidone and its active metabolite 9-hydroxy risperidone. That THC treatment equivalently reduced the brain concentrations of both risperidone and 9-hydroxy risperidone argues against THC inducing CYP450 enzymes, as otherwise one might expect a reduction in the risperidone concentration to coincide with an increased 9-hydroxy risperidone concentration. Furthermore, CYP2D6 is the major enzyme responsible for risperidone metabolism in humans and there is no available evidence to suggest it or its mouse orthologs are inducible by xenobiotics (Miksys et al, 2005; Murray, 2006; Nelson et al, 2004; Sheehan et al, 2010; Urichuk et al, 2008).
This raised the possibility that P-gp might play a role, as it is inducible and its localization at the BBB has been shown to strongly limit the brain accumulation of drug substrates by transporting them from the brain into the peripheral blood supply (Boulton et al, 2002; Doran et al, 2005; Linnet and Ejsing, 2008). Risperidone and its active metabolite 9-hydroxy risperidone are excellent substrates of P-gp (Boulton et al, 2002; Brzozowska et al, 2016; Doran et al, 2005; Wang et al, 2004), and this we confirmed here by showing that P-gp KO mice attained significantly higher brain concentrations of these drugs. The much greater effect of P-gp KO on brain compared with plasma concentrations of risperidone and its metabolite support the viewpoint that P-gp localized at the BBB plays a dominant role in risperidone and 9-hydroxy risperidone brain uptake.
Induction of P-gp at the BBB might then explain the reduced brain concentrations of risperidone and 9-hydroxy risperidone we observed. Indeed, mice that underwent the THC pretreatment regimen showed markedly increased P-gp expression in the brain 2 weeks following their final THC exposure. The distribution pattern of staining is consistent with localization on brain microvessels, where P-gp is preferentially expressed in the brain (Bendayan et al, 2006; Gazzin et al, 2008). This also confirms our observations that repeated THC exposure in rats significantly increased P-gp mRNA expression in brain endothelial cells selectively captured using laser-capture microdissection (data not shown). It is also noteworthy that THC-induced P-gp upregulation was observed in the ventrolateral septum, nucleus accumbens, and the paraventricular nucleus of the thalamus, three brain regions where THC reversed risperidone-induced c-Fos expression, and regions implicated in antipsychotic drug action (Fujimura et al, 2000; Sumner et al, 2004). Our findings also accord with schizophrenia patients having region-specific enhancement of P-gp activity (De Klerk et al, 2010, 2011). A positron emission topography (PET) study showed chronic schizophrenia patients had considerably lower brain uptake of the P-gp substrate 11C-verapamil in the temporal cortex, basal ganglia, and amygdala, but not in the thalamus or prefrontal cortex. These results overlap somewhat with our findings that showed THC treatment increased P-gp expression in the basal ganglia but not in the prefrontal cortex. Our results therefore further support the notion that pharmacoresistance in schizophrenia might be explained by enhanced P-gp expression lowering the brain accumulation of antipsychotic drugs. Furthermore, our study implies that cannabis use might be responsible for the enhanced brain P-gp activity observed in schizophrenia patients.
The mechanism responsible for THC-induced upregulation of P-gp could be explored in future studies. Our prior work has shown that incubation of human leukemia cells with THC increased P-gp mRNA that was mediated by cannabinoid CB2 receptors (Arnold et al, 2012; Holland et al, 2006). CB2 receptors are expressed on brain endothelial cells in both mice and humans (Golech et al, 2004), and therefore may play a role here. It is conceivable that THC activation of CB2 receptors could further activate intracellular signaling pathways that affect nuclear transcription factors involved in the induction of P-gp (Mestre et al, 2006). Interestingly, several nuclear transcription factors regulating P-gp transporter expression such as constitutive androstane receptor (CAR), pregnane X receptor (PXR), and nuclear factor-E2-related factor 2 (Nrf2) are activated by cannabinoids (Downer et al, 2012; Fakhfouri et al, 2012; Juknat et al, 2012).
Taken together, our results suggest that THC exposure decreased the neurobehavioral effects of risperidone because of P-gp upregulation in the brain and consequent reductions in risperidone and 9-hydroxy risperidone brain concentrations. As clozapine is not a good substrate of P-gp (Boulton et al, 2002; Doran et al, 2005), we hypothesized that the neurobehavioral effects of clozapine might not be susceptible to THC preexposure. Indeed, the same THC treatment regimen did not influence the effects of clozapine on c-Fos expression in the brain, PPI, or locomotor activity. It is noteworthy that risperidone and clozapine share a very similar receptor-binding profile, and risperidone was developed to mimic the combined D2/5-HT2A receptor antagonist profile of clozapine (Meltzer et al, 1989; Schotte et al, 1996). Although we showed no effect of THC on D2 and 5-HT2A receptor binding, we cannot definitively rule out a pharmacodynamic mechanism, as there remains the possibility that THC affected a receptor system or biased intracellular signaling pathway specific to the actions of risperidone and not clozapine. Despite this, it is clear that the differential P-gp binding characteristic of risperidone and clozapine may offer an explanation for why THC treatment reduced the neurobehavioral effects of risperidone but not clozapine.
Clozapine is the drug of choice for treatment-resistant patients, and is often used in cannabis-using patients, but mainly from the perspective that it helps to promote cannabis abstinence (Green et al, 2003; McEvoy et al, 2006). Our results suggest that clozapine may also be a favorable drug for cannabis-using schizophrenia patients as it retains its efficacy, unlike other antipsychotic drugs that may be prone to treatment resistance in this population. Rapid stabilization of the patient on antipsychotic drugs is of the utmost importance to the clinical management of schizophrenia as it predicts patient prognosis (Agid et al, 2003; Kinon et al, 2010; Stauffer et al, 2011). However, currently clozapine is only administered to treatment-resistant schizophrenics after at least two antipsychotic trials have been attempted (Kreyenbuhl et al, 2010; Moore et al, 2007). More commonly, there are major delays in initiating clozapine treatment, with long-standing use of combination polypharmacy before commencement with the drug (Howes et al, 2012). Our findings suggest that first-line use of clozapine in cannabis-using FEP schizophrenia patients might be examined as a strategy to improve patient prognoses. Of course, before such a strategy is introduced, our mice findings need to be translated to humans. Given clozapine’s poor toxicity profile, other antipsychotic drugs might be developed in this context. For example, the antipsychotic drug blonanserin could be explored (Kishi et al, 2013; Yang et al, 2010), as it has no affinity for P-gp and has a better safety profile than clozapine (Inoue et al, 2012). In addition, although not reported in the published scientific literature, FDA drug approval submissions suggest other recently introduced antipsychotic drugs are not P-gp substrates, ie, asenapine, brexpiprazole, pimavanserin, iloperidone, and cariprazine.
Here we provide evidence for the first time that prior exposure to the most abundant phytocannabinoid constituent in cannabis, THC, reduces the neurobehavioral effects of the commonly used antipsychotic drug risperidone. This occurred because of THC reducing the brain disposition of risperidone and its active metabolite 9-hydroxy risperidone. This finding appeared to be explained by THC inducing P-gp in the brain, as both risperidone and its active metabolite are excellent P-gp substrates. The neurobehavioral effects of the non-P-gp substrate clozapine, the drug of choice for treatment-resistant patients, were unaffected by THC exposure. This suggests that differential P-gp binding may be an important consideration for antipsychotic drug selection for treatment-resistant patients with a cannabis use history. Our results may also help explain the long-standing mystery of the selective efficacy of clozapine in treatment-resistant schizophrenia patients.
Funding and disclosure
This work was supported by a University of Sydney Bridging Grant and a Bosch Translational Grant-in-Aid. It was also supported by a NARSAD Young Investigator Award. The authors declare no conflict of interest.
References
Agid O, Kapur S, Arenovich T, Zipursky RB (2003). Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 60: 1228–1235.
Arnold JC, Hone P, Holland ML, Allen JD (2012). CB2 and TRPV1 receptors mediate cannabinoid actions on MDR1 expression in multidrug resistant cells. Pharmacol Rep 64: 751–757.
Bendayan R, Ronaldson PT, Gingras D, Bendayan M (2006). In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem 54: 1159–1167.
Boucher AA, Hunt GE, Karl T, Micheau J, McGregor IS, Arnold JC (2007). Heterozygous neuregulin 1 mice display greater baseline and Delta(9)-tetrahydrocannabinol-induced c-Fos expression. Neuroscience 149: 861–870.
Boucher AA, Hunt GE, Micheau J, Huang X, McGregor IS, Karl T et al (2011). The schizophrenia susceptibility gene neuregulin 1 modulates tolerance to the effects of cannabinoids. Int J Neuropsychopharmacol 14: 631–643.
Boulton DW, DeVane CL, Liston HL, Markowitz JS (2002). In vitro P-glycoprotein affinity for atypical and conventional antipsychotics. Life Sci 71: 163–169.
Brzozowska N, Li KM, Wang XS, Booth J, Stuart J, McGregor IS et al (2016). ABC transporters P-gp and Bcrp do not limit the brain uptake of the novel antipsychotic and anticonvulsant drug cannabidiol in mice. PeerJ 4: e2081.
Csomor PA, Preller KH, Geyer MA, Studerus E, Huber T, Vollenweider FX (2014). Influence of aripiprazole, risperidone, and amisulpride on sensory and sensorimotor gating in healthy 'low and high gating' humans and relation to psychometry. Neuropsychopharmacology 39: 2485–2496.
De Klerk OL, Bosker FJ, Luurtsema G, Nolte IM, Dierckx R, Den Boer JA et al (2011). The role of p-glycoprotein in psychiatric disorders: a reliable guard of the brain? Cent Nerv Syst Agents Med Chem 11: 197–209.
De Klerk OL, Willemsen AT, Bosker FJ, Bartels AL, Hendrikse NH, den Boer JA et al (2010). Regional increase in P-glycoprotein function in the blood-brain barrier of patients with chronic schizophrenia: a PET study with [11C] verapamil as a probe for P-glycoprotein function. Psychiatry Res 183: 151–156.
Doran A, Obach RS, Smith BJ, Hosea NA, Becker S, Callegari E et al (2005). The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: evaluation using the MDR1A/1B knockout mouse model. Drug Metab Dispos 33: 165–174.
Downer EJ, Clifford E, Amu S, Fallon PG, Moynagh PN (2012). The synthetic cannabinoid R(+)WIN55,212-2 augments interferon-beta expression via peroxisome proliferator-activated receptor-alpha. J Biol Chem 287: 25440–25453.
Fakhfouri G, Ahmadiani A, Rahimian R, Grolla AA, Moradi F, Haeri A (2012). WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-gamma pathway. Neuropharmacology 63: 653–666.
Fibiger H (1994). Neuroanatomical targets of neuroleptic drugs as revealed by Fos immunochemistry. J Clin Psychiatry 55 (Suppl B): 33–36.
Food and Drug Administration (2005) Estimating the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers. Food and Drug Administration: Rockville, MD, USA.
Fujimura M, Hashimoto K, Yamagami K (2000). The effect of the antipsychotic drug mosapramine on the expression of Fos protein in the rat brain: comparison with haloperidol, clozapine and risperidone. Life Sci 67: 2865–2872.
Gazzin S, Strazielle N, Schmitt C, Fevre-Montange M, Ostrow JD, Tiribelli C et al (2008). Differential expression of the multidrug resistance-related proteins ABCb1 and ABCc1 between blood-brain interfaces. J Comp Neurol 510: 497–507.
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001). Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade inreview. Psychopharmacology 156: 117–154.
Glennon RA, Titeler M, McKenney J (1984). Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci 35: 2505–2511.
Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R et al (2004). Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res 132: 87–92.
Green AI, Burgess ES, Dawson R, Zimmet SV, Strous RD (2003). Alcohol and cannabis use in schizophrenia: effects of clozapine vs. risperidone. Schizophr Res 60: 81–85.
Gunasekaran N, Long L, Dawson B, Hansen G, Richardson D, Li K et al (2009). Reintoxication: the release of fat-stored Δ9-tetrahydrocannabinol (THC) into blood is enhanced by food deprivation or ACTH exposure. Br J Pharmacol 158: 1330–1337.
Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ et al (2015). Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol. 25: 325–334.
Holland ML, Panetta JA, Hoskins JM, Bebawy M, Roufogalis BD, Allen JD et al (2006). The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistantcells. Biochem Pharmacol 71: 1146–1154.
Howes OD, Vergunst F, Gee S, McGuire P, Kapur S, Taylor D (2012). Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry 201: 481–485.
Inoue T, Katsumata T, Yabuki M, Yamaguchi N, Osada K, Tagawa M et al (2012). Blonanserin, a novel atypical antipsychotic agent not actively transported as substrate by P-glycoprotein. Prog Neuropsychopharmacol Biol Psychiatry 39: 156–162.
Joober R, Boksa P (2010). Clozapine: a distinct, poorly understood and under-used molecule. J Psychiatry Neurosci 35: 147–159.
Juknat A, Pietr M, Kozela E, Rimmerman N, Levy R, Coppola G et al (2012). Differential transcriptional profiles mediated by exposure to the cannabinoids cannabidiol and Delta(9)-tetrahydrocannabinol in BV-2 microglial cells. Br J Pharmacol 165: 2512–2528.
Kapur S, Remington G (2001). Dopamine D2 receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry 50: 873–883.
Kapur S, Zipursky R, Jones C, Remington G, Houle S (2000). Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 157: 514–520.
Kinon BJ, Chen L, Ascher-Svanum H, Stauffer VL, Kollack-Walker S, Zhou W et al (2010). Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology 35: 581–590.
Kishi T, Matsuda Y, Nakamura H, Iwata N (2013). Blonanserin for schizophrenia: systematic review and meta-analysis of double-blind, randomized, controlled trials. J Psychiatr Res 47: 149–154.
Koskinen J, Löhönen J, Koponen H, Isohanni M, Miettunen J (2010). Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull 36: 1115–1130.
Kreuz DS, Axelrod J (1973). Delta-9-tetrahydrocannabinol: localization in body fat. Science 179: 391–393.
Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB (2010). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull 36: 94–103.
Li L, Du Y, Li N, Wu X, Wu Y (2009). Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neurosci Biobehav Rev 33: 1157–1167.
Linnet K, Ejsing TB (2008). A review on the impact of P-glycoprotein on the penetration of drugs into the brain. Focus on psychotropic drugs. Eur Neuropsychopharmacol 18: 157–169.
Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P (2014). Prognosis of schizophrenia in persons with and without a history of cannabis use. Psychol Med 44: 2513–2521.
McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA et al (2006). Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 163: 600–610.
Meltzer HY, Matsubara S, Lee J (1989). Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther 251: 238–246.
Mestre L, Correa F, Docagne F, Clemente D, Guaza C (2006). The synthetic cannabinoid WIN 55,212-2 increases COX-2 expression and PGE(2) release in murine brain-derived endothelial cells following Theiler's virus infection. Biochem Pharmacol 72: 869–880.
Miksys SL, Cheung C, Gonzalez FJ, Tyndale RF (2005). Human CYP2D6 and mouse CYP2Ds: organ distribution in a humanized mouse model. Drug Metab Dispos 33: 1495–1502.
Montanari F, Ecker GF (2015). Prediction of drug-ABC-transporter interaction—recent advances and future challenges. Adv Drug Deliv Rev 86: 17–26.
Moons T, de Roo M, Claes S, Dom G (2011). Relationship between P-glycoprotein and second-generation antipsychotics. Pharmacogenomics 12: 1193–1211.
Moore TA, Covell NH, Essock SM, Miller AL (2007). Real-world antipsychotic treatment practices. Psychiatr Clin North Am 30: 401–416.
Murray M (2006). Role of CYP pharmacogenetics and drug‐drug interactions in the efficacy and safety of atypical and other antipsychotic agents. J Pharm Pharmacol 58: 871–885.
Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW (2004). Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14: 1–18.
Nichols DE (2004). Hallucinogens. Pharmacol Ther 101: 131–181.
Ouagazzal AM, Jenck F, Moreau JL (2001). Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity? Psychopharmacology 156: 273–283.
Patel R, Wilson R, Jackson R, Ball M, Shetty H, Broadbent M et al (2016). Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open 6: e009888.
Quednow BB, Wagner M, Westheide J, Beckmann K, Bliesener N, Maier W et al (2006). Sensorimotor gating and habituation of the startle response in schizophrenic patients randomly treated with amisulpride or olanzapine. Biol Psychiatry 59: 536–545.
Reagan-Shaw S, Nihal M, Ahmad N (2008). Dose translation from animal to human studies revisited. FASEB J 22: 659–661.
Remington G, Lee J, Agid O, Takeuchi H, Foussias G, Hahn M et al (2016). Clozapine's critical role in treatment resistant schizophrenia: ensuring both safety and use. Expert Opin Drug Saf 15: 1193–1203.
Schotte A, Janssen P, Gommeren W, Luyten W, Van Gompel P, Lesage A et al (1996). Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology 124: 57–73.
Sheehan J, Sliwa J, Amatniek J, Grinspan A, Canuso C (2010). Atypical antipsychotic metabolism and excretion. Curr Drug Metab 11: 516–525.
Spiro AS, Wong A, Boucher AA, Arnold JC (2012). Enhanced brain disposition and effects of Delta9-tetrahydrocannabinol in P-glycoprotein and breast cancer resistance protein knockout mice. PLoS ONE 7: e35937.
Stauffer VL, Case M, Kinon BJ, Conley R, Ascher-Svanum H, Kollack-Walker S et al (2011). Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res 187: 42–48.
Sumner BE, Cruise LA, Slattery DA, Hill DR, Shahid M, Henry B (2004). Testing the validity of c-fos expression profiling to aid the therapeutic classification of psychoactive drugs. Psychopharmacology (Berl) 171: 306–321.
Swift W, Wong A, Li KM, Arnold JC, McGregor IS (2013). Analysis of cannabis seizures in NSW, Australia: cannabis potency and cannabinoid profile. PLoS ONE 8: e70052.
Todd SM, Arnold JC (2016). Neural correlates of cannabidiol and Delta9-tetrahydrocannabinol interactions in mice: implications for medical cannabis. Br J Pharmacol 173: 53–65.
Urichuk L, Prior TI, Dursun S, Baker G (2008). Metabolism of atypical antipsychotics: involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr Drug Metab 9: 410–418.
Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR et al (2008). Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend 94: 191–198.
Wang JS, Ruan Y, Taylor RM, Donovan JL, Markowitz JS, DeVane CL (2004). The brain entry of risperidone and 9-hydroxyrisperidone is greatly limited by P-glycoprotein. Int J Neuropsychopharmacol 7: 415–419.
Yang J, Kim Y-H, Kwon J-S, Lee S-Y, Lee S-H, Yi J-S et al (2010). Efficacy and tolerability of blonanserin in the patients with schizophrenia: a randomized, double-blind, risperidone-compared trial. Clin Neuropharmacol 33: 169–175.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Brzozowska, N., de Tonnerre, E., Li, K. et al. The Differential Binding of Antipsychotic Drugs to the ABC Transporter P-Glycoprotein Predicts Cannabinoid–Antipsychotic Drug Interactions. Neuropsychopharmacol 42, 2222–2231 (2017). https://doi.org/10.1038/npp.2017.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2017.50
This article is cited by
-
Herb-anticancer drug interactions in real life based on VigiBase, the WHO global database
Scientific Reports (2022)