Abstract
In people with a prior history of opioid misuse, cues associated with previous drug intake can trigger relapse even after years of abstinence. Examining the processes that lead to the formation and maintenance of the memories between cues/context and the opioid may help to discover new therapeutic candidates to treat drug-seeking behavior. The hippocampus is a brain region essential for learning and memory, which has been involved in the mechanisms underlying opioid cravings. The formation of memories and associations are thought to be dependent on synaptic strengthening associated with structural plasticity of dendritic spines. Here, we assess how dendritic spines in the CA1 region of the hippocampus are affected by morphine-conditioning training. Our results show that morphine pairing with environmental cues (ie, the conditioned place preference (CPP) apparatus) triggers a significant decrease in the number of thin dendritic spines in the hippocampus. Interestingly, this effect was observed regardless of the expression of a conditioned response when mice were trained using an unpaired morphine CPP design and was absent when morphine was administered in the home cage. To investigate the mechanism underlying this structural plasticity, we examined the role of Rho GTPase in dendritic spine remodeling. We found that synaptic expression of RhoA increased with morphine conditioning and blocking RhoA signaling prevented the expression of morphine-induced CPP. Our findings uncover novel mechanisms in response to morphine-associated environmental cues and the underlying alterations in spine plasticity.
Similar content being viewed by others
INTRODUCTION
Opioid abuse is an emerging economic and social problem in the United States with 11 million people reporting the non-medical use of opioid analgesics (Compton and Volkow, 2006a, b). Chronic exposure to opioids and other drugs of abuse induce the formation and maintenance of maladaptive drug cue–context associations that can drive craving and relapse (O'Brien et al, 1992; Daglish et al, 2001; Crombag et al, 2008; Napier et al, 2013). Recent evidence shows that development of morphine-induced conditioned place preference (CPP) requires dopaminergic transmission from the ventral tegmental area to the hippocampus (Esmaeili et al, 2012), and that drug–context associations result in receptor adaptations and alterations in neuronal function in the hippocampus (Moron et al, 2010). In our previous studies, we used the context-dependent behavioral sensitization model where repeated morphine administered specifically in the context of the locomotor activity chamber, and not at the home cage, resulted in a sensitized locomotor response when re-exposed to lower doses of morphine. This effect was mediated by an increase in synaptic GluA1 AMPA receptor expression and transmission in the hippocampus (Xia et al, 2011; Fakira et al, 2014). Following these initial studies, we conducted additional studies using the morphine CPP model following the same schedule of morphine administration (Portugal et al, 2014). As previously found using the context-dependent sensitization model, the morphine CPP model also resulted in alterations in glutamatergic transmission and impairment in hippocampal long-term potentiation at CA1 synapses (Xia et al, 2011; Fakira et al, 2014; Portugal et al, 2014). Altogether, these studies clearly define a central role for the hippocampus in the association between the context and morphine exposure. However, it is currently unknown whether this impacts synaptic plasticity at the structural level.
Dendritic spines are the structural units of synaptic function and plasticity, and examining changes in spine morphology can elucidate essential signaling pathways that are involved in the remodeling of synapses (Nishiyama and Yasuda, 2015). Three main types of spines are present in the hippocampus: thin, mushroom, and stubby (Bourne and Harris, 2007; Bourne and Harris, 2008). Thin spines (~65% of the population) have a long narrow neck and are composed of small post-synaptic densities containing NMDA receptors and few AMPA receptors. Thin spines form and retract rapidly with synaptic activity. Mushroom spines (~25%) have a narrow neck and large head whose post-synaptic densities contain AMPA and NMDA receptors (Matsuzaki et al, 2001). These spines are more structurally stable, and contain machinery for calcium regulation (Hering and Sheng, 2001; Bourne and Harris, 2007). Stubby spines (~10%) have a short neck, therefore, signaling at these synapses is not compartmentalized by a narrow neck as in thin and mushroom spines. Structural plasticity of dendritic spines is controlled by actin polymerization. NMDA-CamKII-dependent activation of small GTPases, including RhoA and Rac1, ultimately regulates the activity of the actin severing protein cofilin (Hering and Sheng, 2001; Harvey et al, 2008; Nakazawa et al, 2008; McNair et al, 2010; Christoffel et al, 2011; Murakoshi et al, 2011; Golden et al, 2013; Wang et al, 2013b).
Although previous studies investigated the effects of morphine on spine density, the impact of morphine CPP on hippocampal spine remodeling and the underlying signaling mechanisms is unknown. Morphine self-administration decreases the overall spine density in the hippocampus and nucleus accumbens (Robinson et al, 2002). In addition, in vitro studies aimed at investigating the direct effect of chronic morphine on cultured hippocampal neurons elucidated similar decreases in spine density (Liao et al, 2005; Liao et al, 2007; Zheng et al, 2010; Miller et al, 2012). Until now, only a few studies characterizing the effects of opioids on spine morphology have been conducted. However, the majority of these studies rely on manual counts of two dimensional images that exhibit a selective bias against thin spines (Shen et al, 2009; Dumitriu et al, 2012). In addition, the role of Rho GTPase signaling in opioid-induced structural plasticity has not been investigated yet. Moreover, the impact that the observed impaired synaptic plasticity, following context-dependent sensitization to morphine and morphine CPP, has on structural plasticity has not been investigated. Furthermore, given the fact that alterations in synaptic plasticity may affect other types of memory (ie, spatial memory), it is necessary to know whether morphine-induced alterations in plasticity may also affect other types of memory such as spatial memory. Therefore, the goals of this study are to determine whether morphine conditioning paired with environmental cues (ie, the CPP apparatus) is sufficient to trigger remodeling of dendritic spines in the hippocampus and whether this effect is associated with alterations in spatial memory. Finally, we examine whether the Rho GTPase signaling pathway mediates this alteration in dendritic spine structural plasticity that may be responsible for morphine-induced CPP.
MATERIALS AND METHODS
Animals and Morphine Treatment
Adult (6–8 weeks) male C57BL/6 mice (Harlan; n=120 mice) maintained on a 12 h light/dark cycle with water and food ad libitum were used in this study. Morphine sulfate (provided by the National Institute on Drug Abuse) was dissolved in sterile saline at a concentration of 1 mg/ml. For all experiments, mice received daily subcutaneous (s.c.) injections of escalating doses of morphine (5 (Day 1), 8 (Day 2), 10 (Day 3) and 15 (Day 4) mg/kg; see Figures 2a, 4a and 5a) or equivalent volume saline in three different paradigms: paired CPP, unpaired CPP, and home cage treatments. All protocols were approved by the IACUC at Columbia University and Washington University in St Louis, according to NIH’s Guide for the Care and Use of Laboratory Animals.
Conditioned Place Preference (CPP)
Apparatus
Training and testing of morphine conditioning following a paired and unpaired CPP occurred in a 3-chamber CPP apparatus as we previously reported (Portugal et al, 2014). Throughout the manuscript, the term ‘morphine conditioning paired with environmental cues’ refers to all mice that received morphine in the context of the CPP chamber. The neutral center chamber is 12 cm long, with smooth white PVC plastic walls and floor, whereas the two conditioning chambers are 28 cm long and have different visual cues on the walls (vertical vs horizontal black and white stripes), and black PVC plastic floors lined with woodchip bedding. The chambers are separated by manual guillotine doors to allow access to all three chambers, and photobeam arrays in all three chambers will be controlled by Med-PC software (Med-associates, St Albans, VT) to detect preference for each chamber.
Morphine conditioning following a paired CPP design
On the pre-conditioning day (Day 0), mice were placed in the neutral chamber and preference for all three chambers was measured for 15 min. Following an unbiased design, mice were randomly assigned to one of the two chambers and to the morphine- and saline-treated groups. During training (Days 1–4), mice received s.c. saline and were placed in the saline-paired chambers in the morning for 30 min. During the afternoon (4 h later), mice received morphine s.c. in the morphine-paired compartment for 30 min. In these experiments, escalating doses of morphine were used for each conditioning session (5 (Day 1), 8 (Day 2), 10 (Day 3) and 15 (Day 4) mg/kg; Figures 2a, 4a and 5a) as this dosing regimen has been shown to produce robust morphine CPP (Portugal et al, 2014). Saline control animals were treated with saline s.c. in both conditioning chambers. Mice received a preference test on day 5 when they were placed in the central compartment and allowed to freely explore the apparatus for 15 min. All experiments were counterbalanced between the vertical and horizontal pairings. Preference was measured both by comparing the percentage of time they spent in the conditioning chambers prior to training and after training and using a Wilcoxon signed ranked test to determine whether mice spent significantly greater than 50% of time in the morphine-paired chamber post conditioning.
Morphine conditioning following an unpaired CPP design
On the pre-conditioning day (Day 0), mice were placed in the neutral chamber and preference for all three chambers was measured for 15 min. Mice were randomly assigned to the morphine- and saline-treated groups in an unbiased manner. In this design, morphine-pairing was alternated every day such that if they received p.m. dose of morphine in the vertical chamber on day 1, on day 2, they would receive the p.m. dose of morphine in the horizontal chamber etc. Ultimately, the mice received two morphine pairings in the horizontal chamber and two pairings in the vertical chamber. Experiments were counterbalanced so that half the mice started with the vertical chambers and ended with the horizontal and the other half started with the horizontal chambers and ended with the vertical chambers. In this manner, the 5 (Day 1), 8 (Day 2), 10 (Day 3) and 15 (Day 4) mg/kg doses (Figure 2a) were randomized between the chambers. Mice received a preference test on day 5 when they were placed in the central compartment and allowed to freely explore the apparatus for 15 min. Preference was measured both by comparing the percentage of time they spent in the conditioning chambers prior to training and after training and using a Wilcoxon signed ranked test to determine whether mice spent significantly greater than 50% of time in either the vertical, horizontal, or center chamber post conditioning. Data were analyzed in the same way as in the morphine-paired CPP design.
Home Cage Morphine Treatment
In the a.m. session, all mice received saline and were placed directly in their home cage. Four hours later, mice were injected s.c. with either escalating doses of morphine (5 (Day 1), 8 (Day 2), 10 (Day 3) and 15 (Day 4) mg/kg) or equivalent volume of saline and placed directly into their home cages.
Tissue Collection
Brains were collected immediately following preference testing for paired and unpaired groups or day 5 for the home cage group. The hippocampus was dissected out and immediately frozen on dry ice for biochemical experiments or the mice were transcardially perfused with 4%PFA and brains post-fixed for 24 h for spine imaging experiments.
Spine Analysis
Two weeks prior to pre-conditioning (Day 0), 0.5 μl of AAV5-CamKII-eYFP virus (5 × 1011 particles per ml) were infused into the dorsal hippocampus (A/P:-1.7, Lat: +/− 1.5, D/V:-1.8) unilaterally so that YFP was expressed in pyramidal neurons. This injection was counterbalanced so that both hemispheres were represented in the analysis. To visually enhance virally delivered YFP expression, we completed immunohistochemical analysis in 200 μm coronal brain slices with chicken anti-GFP (primary antibody, Aves Labs, Tigard, OR) and anti-chicken 488 (secondary antibody, Molecular Probes, Eugene, OR). Staining for GFP served as a method to increase the brightness of the viral YFP in order to easily image small sub-micron dendritic spines, a method that has been used by others (Wang et al, 2013a). To qualify for spine analysis, dendritic segments had to satisfy the following requirements: (i) the segment had to be completely filled (all endings were excluded) and (ii) segment must be at least 50 μm from the soma. Dendritic segments were imaged using a Nikon Ti Eclipse Confocal microscope with a × 60 oil immersion objective (N.A.=1.4). Image acquisition was as follows; pixel size in the x-y plane: 0.03 μm, z-stacks: 0.1 μm steps, resolution: 1024 × ~256, pixel dwell time: 2 μm/s, line average: 4. Images were deconvoluted with Volocity software to improve contrast and resolution. NeuronStudio software (Mount Sinai, New York, NY) with the rayburst algorithm was used to quantify spine size, shape, and volume as per Dumitriu et al (2011). Approximately 3–6 dendritic segments (dendritic length per segment: 30 μm) per neuron were analyzed from 3–6 neurons per animal in 4–6 animals per group. The experimenter obtaining and analyzing confocal images was blinded to treatment groups. Throughout the course of these experiments, there were a few failures, meaning there was no labeling of CA1 neurons. In these cases, there was a needle track but no labeling indicating a failure of the virus to expel from the needle or less likely because of unintended infusion into the incorrect brain region.
Synaptosomal and Post-Synaptic Density Fractionation
Hippocampi were collected from both hemispheres and pooled. The synaptosomal and the post-synaptic density fractions were obtained as previously described (Xia et al, 2011; Fakira et al, 2014; Portugal et al, 2014).
Western Blotting
Protein concentrations were measured using BCA protein assay as per the manufacturer’s specifications. The total homogenate and post-synaptic density fractions were loaded equally (10–30 μg) and separated on 12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. After blocking in 5% non-fat dry milk in TBS containing 0.1% Tween-20, membranes were incubated with RhoA (1 : 1000, Cell Signaling, Danvers, MA), Rac1 (1 : 1000, Millipore, Darmstadt, Germany), or actin (1 : 5000; Millipore, Darmstadt, Germany) antibody overnight at 4 °C. Membranes were then probed with anti-rabbit-HRP 1 : 5000 followed by Amersham ECL Prime detection or IRDye 680 anti-rabbit/ IRDye 800 anti-mouse (LI-COR, Lincoln, NE) and imaged using Odyssey software. After obtaining RhoA staining, blots were stripped and re-probed for Rac1 and then stripped and re-probed for actin. The intensity of each sample’s RhoA or Rac1 band and its respective beta-actin was measured using ImageJ software. The intensity of these bands was normalized to its beta-actin and represented as a percent of the average intensity of the saline control samples.
Barnes Maze
In order to determine whether morphine conditioning training may impact other types of learning and memory, we conducted additional studies using the Barnes Maze, a model to examine spatial memory in rodents. The Barnes maze (Stoeling, Wood Dale, IL) is an elevated circular platform consisting of a 91 cm diameter open field with 20 equidistant holes around the perimeter. The maze contains one escape hole, a dark recessed chamber in which the mouse can hide, and 19 other 5-cm (diameter) deep chambers, which are too small for the animal to escape the open field. Visual cues placed on each wall of the room allowed spatial navigation while white noise was playing during the trial. This noise turned off automatically when mice entered the escape hole. For Barnes maze habituation, performed after the preconditioning test for CPP (Day 0), mice were placed in the center of the platform and given 180 s to enter the escape hole. Each mouse was given two trials separated by 2-min intervals. If the mouse did not enter the escape hole at the end of 180 s, they were gently placed into the hole and remained there for 1 min. On training (Days 1–4), mice were given three 180-s trials on the Barnes maze separated by 15-min intervals after morning saline conditioning in the CPP paradigm (Figure 5a). Latency to enter the escape hole was recorded using Any Maze software. On Day 5, mice were given a probe test on the Barnes maze 1 h after the CPP post-conditioning test. During this probe test, the escape hole was replaced with a shallow chamber in which the mice cannot enter. Animals were placed in the center of the platform for a 90-s trial. Latency to first entry and number of entries into the target hole were recorded.
Cannulae Implantation and RhoA/Rho-associated Kinase (ROCK) Inhibitor Experiments
Three weeks prior to morphine-paired CPP training mice were anesthetized using isofluorane, a craniotomy was performed, and two cannulae (C315G/SPC, 1.5 mm; Plastics One, Roanoke, VA) were lowered bilaterally into the hippocampus (−1.7 mm A/P; −/+1.5 mm Lat; −0.8 mm D/V from bregma) using a stereotaxic frame. The cannulae were secured using two bone screws and affixed with dental cement (Lang Dental, Wheeling, IL). After pre-conditioning (Day 0), mice were separated into two groups: (i) morphine-paired CPP–intra hippocampal vehicle and (ii) morphine paired CPP–intra hippocampal ROCK inhibitor, H1152 (Tocris, Minneapolis, MN). H-1152 is a potent and selective inhibitor of ROCK (Ki 1.6 nM) with low activity at other kinases (Ikenoya et al, 2002). On training days 1–4, 0.5 μl of either vehicle or H1152 (0.02 or 0.14 ng in 0.5 μl injected per side) was infused into the hippocampus through the internal cannula using an infusion pump (World Precision Instruments, Sarasota, FL). Mice were returned to their home cages for 15–20 min prior to conditioning session.
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism 6 software. For behavioral experiments, a two-way ANOVA followed by Bonferroni post hoc tests, when applicable, were used to compare animal’s performances. Wilcoxon rank test compared to 50% was also used to assess animal’s preference for the drug-paired compartment. For spine analysis, a two-way ANOVA followed by Sidak's multiple comparisons post hoc tests were used to compare treatment groups. For western blot analysis, an unpaired two-tailed Student t-test was used to assess statistical differences in the band intensity normalized to their beta-actin internal control. Statistical difference was considered with a p<0.05 in all experiments.
RESULTS
Viral Transfection to Analyze Dendritic Spine Morphology
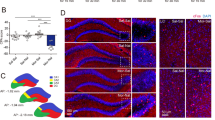
In order to examine structural plasticity of dendritic spines following morphine conditioning under both paired and unpaired CPP design (or following home cage morphine treatment), we optimized a protocol that combines viral transfection with imaging using 3D reconstruction, which will allow for full characterization of spines types, previously only possible with serial section electron microscopy. As described above, mice were injected with AAV5 constructs expressing YFP under CaMKII promoter into the dorsal hippocampus to label pyramidal neurons (Figure 1a). Following brain fixation, slices were obtained and CA1 pyramidal neurons were imaged using high resolution confocal microscopy. Figure 1b shows that injection of the AAV5-CaMKII-eYFP construct labels the majority of pyramidal neurons in the dorsal hippocampus. For the qualitative and quantitative analysis of dendritic spines, we used high resolution 3D imaging of CA1 dendritic spines and manual spine counting using NeuroStudio software. This approach has recently been shown to improve counts of thin spines compared with studies using 2D methods and Golgi staining techniques (Shen et al, 2009; Dumitriu et al, 2011). Raw images (Figure 1c, panel 1) were deconvoluted with Volocity software to improve contrast and resolution (Figure 1c, panel 2). NeuroStudio software with the rayburst algorithm was used to quantify spine size, shape, and volume (Figure 1c, panels 3 and 4). Data in Figure 1d show that viral injection and imaging techniques allowed us to identify thin, mushroom, and stubby dendritic spines in CA1 hippocampus with similar proportions to those reported previously using electron microcopy approaches (Bourne and Harris, 2007; Bourne and Harris, 2008).
Analysis of hippocampal CA1 dendritic spines. (a) Schematic representation of the site of AAV5-CamKII-eYFP injection. (b) Confocal image of the dorsal hippocampus 2 weeks following stereotaxic injection of AAV5-CamKII-eYFP (left panel) and a higher magnification of dorsal hippocampus showing expression of YFP in individual CA1 pyramidal neurons (right panel). (c) Example of image treatment and quantification of spines using NeuronStudio. (d) Quantification of types of spines found on hippocampal pyramidal neurons identifying thin spines (77%), mushroom (14%), and stubby spines (9%) expressed as % of total number of spines counted.
Morphine Conditioning Paired with Environmental Cues Results in Morphological Alterations in Hippocampal Dendritic Spines
To examine whether morphine conditioning training affects spine morphology and density in CA1 pyramidal neurons, AAV5-CamKII-eYFP was injected into the dorsal hippocampus to label pyramidal neurons. Two weeks later, mice were conditioned following a morphine-paired or unpaired CPP-conditioning paradigm. As expected, in paired CPP experiments (see Figure 2a schematic), morphine conditioning triggered a significant preference for the drug-paired chamber during the post-conditioning test (two-way ANOVA for repeated measurements, interaction between treatment, and time: F1,23=14,59; p=0.0009, Figure 2b). Post hoc Bonferroni analysis confirmed that during the post-conditioning test, morphine-conditioned mice spent significantly more time in the associated conditioning chamber (p<0.01) (Figure 2b). In the unpaired CPP experiments (see Figure 2a schematic), neither the conditioning (F1,12=0.9653, p=0.3452), the treatment (F1,12=0.002990, p=0.9573), or an interaction between these factors (F1,12=0.01677, p=0.89911) had an impact on post-conditioning results (see Figure 2b).
Paired and unpaired morphine CPP decrease the density of thin spines. (a) Schematic representation of the behavioral paradigm used in morphine-paired and -unpaired CPP. (b) Morphine-treated animals develop a preference during post-conditioning test but not saline-treated mice (respectively, paired: n=12 mice and n=13 mice; unpaired: n=7 mice for both groups). Results are represented as percentage of time spent in the morphine-paired compartment during pre- and post-conditioning tests +/− SEM. #p<0.05, **p<0.01, $$p<0.01 (Wilcoxon test compared to 50%). (c) Representative high resolution images of a dendritic segment from morphine- and saline-paired-treated mice in all conditions analyzed. (d–f) Analysis of the different types of spine density in both saline- and morphine-paired (n=6 mice and n=5 mice, respectively), unpaired (n=4 mice and n=4 mice, respectively), and home cage groups (n=4 mice and n=4 mice, respectively). (d) Thin spine density is decreased in morphine-paired and -unpaired mice compared with saline. No difference was observed in home cage saline (n=4 mice)- and morphine (n=4 mice)-treated mice. Results are represented as spine density per micron +/- SEM. *p<0.05, **p<0.01; #p<0.05, and ##p<0.01 compared with morphine home cage. (e and f) Stubby and mushroom densities were not affected by morphine treatment in either paradigm used.
Brains were collected and processed for morphological analysis after the post-conditioning test. Morphine by itself can influence spine plasticity in other brain regions (Kobrin et al, 2015), therefore, in order to assess whether changes in spine density in the CA1 region of hippocampus were due to context exposure and not morphine treatment by itself, we included a group where animals were administered morphine or saline in their home cages. A two-way ANOVA revealed that thin spine density was affected by the paradigm used (paired, unpaired, or home cage; F2,19=5.606, p=0.0122, Figure 2d) and the treatment received (saline or morphine; F1,19=12.26, p=0.0024, Figure 2c and d). Sidak’s multiple comparison post hoc analysis confirmed that morphine given in either paired or unpaired conditioning resulted in decreased thin spine density compared with saline controls (p<0.01 and p<0.05, respectively). Interestingly, post hoc analysis indicated that thin spine density remained unchanged in mice that received morphine in their home cages (p>0.05) (Figure 2d). No significant change in either stubby (two-way ANOVA: paradigm: F2, 17=0.7904, p=0.4697; treatment: F1, 17=0.2748, p=0.6069; interaction: F2, 17=0.8350, p=0.4509) or mushroom (two-way ANOVA: paradigm: F2, 19=0.5530, p=0.5842; treatment: F1, 19=4.203, p=0.0544; interaction: F2, 19=0.04344, p=0.9576) type spines was observed across paradigms or treatment received (Figure 2e and f).
RhoA Signaling Cascade is Enhanced Following Morphine Conditioning Paired with Environmental Cues
The data shown above indicate that morphine conditioning in the CPP apparatus leads to changes in spine density. These modifications may require the activity of small GTPases such as RhoA and Rac1 in CA1 neurons. Therefore, we examined the expression levels of RhoA and Rac1 in both homogenates and synaptosomal fractions from microdissected hippocampi in order to determine whether these proteins may have a role in the observed morphine-induced decrease in thin spines. In hippocampal homogenates, morphine administered in either paired or unpaired CPP paradigms as well as in the home cage did not induce changes in RhoA (two-tailed unpaired t-test: p=0.9887, p=0.6354, and p=0.4871, respectively) or Rac1 expression (two-tailed unpaired t-test: p=0.4919, p=0.0939, and p=0.3981, respectively) (Supplementary Figure S1a, b, and c). However because Rho activity in the synapses would be more likely to regulate spine morphology, we assessed the expression levels of these proteins in hippocampal synaptosomal fractions. We observed that in the synaptosomal fraction, RhoA expression is increased in mice conditioned following paired and unpaired morphine CPP designs compared with saline controls (two-tailed unpaired t-test: p=0.002 and p=0.0161, respectively) (Figure 3a and b) whereas Rac1 expression is increased only in morphine CPP unpaired mice (two-tailed unpaired t-test: p=0.0003) (Figure 3b). Furthermore, no significant changes in either RhoA or Rac1 expression were found in home cage-treated mice (two-tailed unpaired t-test: p=0.7938 and p=0.1670, respectively) (Figure 3c). Altogether, these results indicate that the context in which morphine is administered (ie, the CPP apparatus), rather than drug by itself, results in a specific increase in the synaptic expression of RhoA and not Rac1 leading to activation of the RhoA signaling cascade, which may result in the remodeling of dendritic spines.
Paired and unpaired morphine CPP induced increases in synaptic RhoA expression. (a) Morphine-paired CPP increases RhoA expression in synaptosomal fractions compared with saline (n=7 mice for both groups). (b) Morphine-unpaired CPP increases both RhoA and Rac1 expression in synaptosomal fractions compared with saline (n=6 mice and n=7 mice, respectively). (c) Morphine home cage does not alter RhoA or Rac1 expression in synaptosomal fractions (n=7 mice for both groups). Data are expressed as percentage of saline +/- SEM and *p<0.05; **p<0.01, and ***p<0.001.
Blockade of the Rho Signaling Cascade in the Hippocampus Results in Prevention of Morphine CPP
Our earlier results demonstrated an upregulation in synaptic RhoA in the hippocampus following morphine conditioning in the CPP chamber (in both paired and unpaired designs). This result suggests that enhancement of the RhoA signaling cascade might be one of the mechanisms underlying the observed decrease in thin spines. Therefore, in a next set of studies, we examined whether the blockade of the RhoA signaling cascade locally in the hippocampus has an effect on the development and subsequent expression of morphine place preference. Blockade of the RhoA signaling cascade was accomplished by microinjection of the ROCK inhibitor H1152, given ROCK’s role as a downstream target of RhoA (Hering and Sheng, 2001). To this end, mice were bilaterally cannulated in the dorsal hippocampus and conditioned in an unbiased paired morphine CPP paradigm. Microinjections of either vehicle or H1152 (2 doses: 0.02 ng/0.5 μl or 0.14 ng/0.5 μl per side, intra-hippocampus) were given 20 min before the mice received morphine (s.c) for the conditioning sessions (Figure 4a). This procedure was repeated prior to each morphine conditioning session to prevent ROCK activation during the formation of the association between the context and the drug. We found that bilateral administration of H1152 in the hippocampus at a dose of 0.14 ng/0.5 μl prior to the conditioning sessions prevented the expression of a morphine place preference (two-way ANOVA for repeated measurements: conditioning: F1,25=31.82, p<0.0001 and interaction between conditioning and time: F2,25=6.411, p=0.0057, Figure 4b). Bonferroni post hoc test confirmed that mice injected with 0.14 ng/0.5 μl H1152 did not exhibit a place preference (p>0.05) (Figure 4b). In contrast, we found that mice injected with both vehicle or a lower dose of H1152 (0.02 ng/0.5 μl) still developed a preference for the morphine compartment (p<0.001 and p<0.01, respectively) (Figure 4b). Although the preference observed in mice injected with 0.02 ng/0.5 μl H1152 was attenuated compared with vehicle-injected mice, this reduction in place preference was not significant (Figure 4b). In addition, locomotor activity was measured during the post-conditioning test using beam break count and no significant difference in activity was observed between any dose of H1152 and vehicle-infused mice, demonstrating that the attenuation in preference during the post-conditioning test mediated by the ROCK inhibitor is not attributable to a decrease in animal’s general motor activity (one-way ANOVA, p=0.2249, Figure 4c). These results are in line with our previous findings and indicate that the RhoA signaling pathway in the hippocampus may be responsible for the development of morphine-CPP.
Blocking the RhoA signaling pathway prevents the expression of morphine place preference. (a) Schematic representation of the behavioral paradigm used in morphine-paired CPP and intra-hippocampal infusion of the ROCK inhibitor. (b) Morphine-paired mice treated with H1152 (0.14 ng in 0.5 μl per side) intra-hippocampally prior to each morphine pairing (n=9 mice) do not develop a place preference for the morphine-paired chamber compared with mice treated with intra-hippocampal saline (n=9 mice). Morphine-paired mice treated with H1152 (0.02 ng in 0.5 μl per side) intra-hippocampally prior to each morphine pairing (n=10 mice) still develop a place preference for the morphine-paired chamber compared with control mice (n=9 mice), although this effect is attenuated. Results are represented as percentage of time spent in the morphine compartment pre- and post-conditioning +/− SEM. **p<0.01, ***p<0.001; $$p<0.01 and $$$p<0.001 Wilcoxon compared to 50%. (c) The administration of H1152 in the hippocampus does not impair general activity during the post-conditioning test. Data are expressed in number of beam breaks +/− SEM. (d) Schematic of three sections spanning the target region of cannulae placement.
Morphine Conditioning-Associated Spine Remodeling Does Not Impact Spatial Memory
Because spine density in the dorsal hippocampus has been linked to spatial memory (Moser et al, 1994; Mahmmoud et al, 2015), it was then critical to evaluate whether the observed morphine conditioning-associated decrease in the density of thin spines could impact the formation of overall spatial memory. To this end, mice were simultaneously conditioned using the morphine-paired CPP paradigm and trained in a spatial learning task using the Barnes maze. In order to evaluate the impact that morphine conditioning in the CPP apparatus could have on spatial learning, the Barnes maze and the CPP training were interspersed. To this end, training sessions took place between the morning and the afternoon CPP conditioning session (Figure 5a). Mice trained on the Barnes maze in addition to the experimental protocol did not have any effect on the animals’ ability to develop a preference for the drug-paired compartment (two-way ANOVA: paradigm: F1,16=15.2, p=0.0013 and interaction between treatment and paradigm: F1,16=8.281, p=0.0109; Bonferroni post hoc test p<0.001 pre- vs post-conditioning test) (Figure 5b). In addition, saline- and morphine-treated animals behaved similarly in the acquisition and performance on the Barnes maze task because no effect of time or interaction in between treatment and time was observed (two-way ANOVA: time: F3,16=9.99, p<0.0001 treatment: F1,16=1.286, p=0.2736, interaction time and treatment: F3,16=1.472, p=0.2341, Figure 5c). Furthermore, we found no difference in number of entries or latency to first entry of the target area between saline- and morphine-treated mice during the probe test (unpaired t-test: p=0.5404 and p=0.9475, respectively, Figure 5d and e). Taken together, these results indicate that the decrease in spine density observed following morphine-paired CPP training does not affect overall spatial memory.
Morphine conditioning training does not alter the acquisition or retention of a spatial learning task. (a) Schematic representation of the behavioral paradigm used for morphine conditioning and Barnes maze training. For these experiments, we used both saline- and morphine-conditioned mice (n=8 mice in both groups). (b) Morphine conditioning induces a preference for the drug compartment. Results are represented as percentage of time spent in the morphine compartment pre- and post-conditioning. n.s. p>0.05, ***p<0.001 and $p<0.05 Wilcoxon test compared to 50% as baseline. (c) Acquisition of spatial Barnes maze memory is represented in seconds before first entry and mean time spent in the target hole. No differences were observed between morphine- and saline-treated animals across the days. Results are expressed in seconds +/− SEM. (d and e) Number of entries and latencies to first visit in the target hole during the probe test reveals that mice treated with morphine (mor) enter the target area of the Barnes maze with the same frequency (d) and latency (e) as saline-treated mice (sal). Results are expressed in number of entries in target hole and seconds +/− SEM, respectively.
DISCUSSION
Because persistent and maladaptive context-dependent memories associated with drugs of abuse are thought to be responsible for the reinstatement of drug-seeking behavior, elucidating the mechanisms that underlie these associations may shed light on relapse prevention. Previous investigations in our laboratory highlighted an effect of context-dependent behavioral sensitization to morphine and morphine CPP on synaptic plasticity within the CA1-Schaeffer collateral pathway (Xia et al, 2011; Fakira et al, 2014; Portugal et al, 2014). The observed impact on functional plasticity may be indicative of alterations in the dynamics of spine morphology because repeated synaptic activation (Yagishita et al, 2014) and stimulation paradigms that induce synaptic plasticity alter both the density and shape of dendritic spines (Yuste and Bonhoeffer, 2001; Bourne and Harris, 2011, 2012). However, limited information is available on the impact of morphine conditioning paired with environmental cues on structural spine plasticity within this pathway.
Persistent structural and functional alterations in dendritic spines caused by drugs of abuse have been proposed to mediate the aberrant learning associated with addiction (Robinson et al, 2002). Recent studies have attempted to determine the role of structural plasticity in drug-induced behavior with conflicting results (Russo et al, 2010; Miller et al, 2012), which may result from the complexity of the intracellular signaling mechanisms underlying structural plasticity of dendritic spines caused by drugs of abuse. Opioids decrease dendritic spine density in the hippocampus (Liao et al, 2007; Miller et al, 2012), however, it is not known whether opioid-induced alterations in hippocampal structural plasticity are integral for drug-induced conditioned responses. Therefore, to fully appreciate the morphological adaptations that occur in the hippocampus following opioid conditioning training, we conducted high resolution imaging and 3D reconstruction in combination with biochemical analyses and in vivo pharmacology to elucidate the signaling pathways that are activated following opioid exposure that may have a role in the mechanisms underlying opioid-context associations. First, we confirmed that morphine-induced decreases in spine density are not associated with memory impairment, using spatial and contextual tasks. However, unlike the findings of Robinson et al (2002), our findings indicate that experimenter-administered morphine paired with environmental cues decreases spine density compared with control animals. This discrepancy can be explained by the difference in the animal strain we used (rats vs mice), the paradigm used (self-administration vs CPP), and the time point of spines analysis. In our studies, spines were examined 24 h after the last morphine conditioning in a morphine CPP paradigm, whereas Robinson et al (2002) conducted their studies 1 month following the last morphine self-administration. This marked difference could readily explain the discrepancies in our results.
We have also demonstrated that both paired and unpaired groups of mice showed a decrease in spine density in the CA1 region of the hippocampus, independent of the expression of a place preference. The lack of place preference in the unpaired paradigm does not exclude the possibility that animals have associated the morphine exposure to the context of the conditioning chambers. In this regard, the observed changes in spine density could be associated with the formation of an association between morphine and the environmental cues (ie, the CPP apparatus) rather than an intrinsic effect of the drug by itself, especially because morphine given in the home cage has no effect on spine density. Another possibility is that the alterations in dendritic spines observed following the paired and unpaired morphine CPP could be due to acute conditioned withdrawal effects (Siegel, 1976, 1978; Kelsey et al, 1990). In addition, data presented here showing that inhibition of ROCK during morphine conditioning sessions prevents the expression of morphine CPP indicate that adaptations occurring during these conditioning sessions are critical for the expression of the preference during the post-conditioning test. However, future studies investigating spine dynamics during morphine conditioning using in vivo imaging approaches could shed light on whether this process is a progressive loss of spines with each conditioning session or whether spine remodeling can be further impacted by the exposure to the CPP chambers during the post-conditioning test, which then triggers the retrieval of the memory leading to the expression of a place preference.
Rho GTPases, including RhoA and Rac1, regulate the actin polymerization and therefore spine morphology (Hering and Sheng, 2001; Christoffel et al, 2011; Golden et al, 2013; Wang et al, 2013a; Wang et al, 2013b). On the basis of previous studies (Nakayama et al, 2000; Tashiro et al, 2000), decreases in spine density can result from either increases in RhoA activation or decreases in Rac1 activation. Indeed, previous studies have demonstrated that increases in RhoA can mediate a loss of thin spines (Chen et al, 2013) and that its overexpression and constitutive activation results in decreases in spine density and length (Nakayama et al, 2000; Tashiro et al, 2000). Our current study uncovered a relationship between decreases in thin spine density and increases in synaptic RhoA expression. Moreover, both of these outcomes occurred when morphine was paired with the CPP chamber and not their home cage environment suggesting that RhoA signaling may have a role in forming drug–context associations. We did uncover an increase in synaptic Rac1 expression (~50%), although this effect was only observed in mice that received morphine conditioning in the unpaired design. However, the RhoA increase, which was on the order of 95% in paired and 178% for the unpaired, is greater than the Rac1 increase indicating that the increase in RhoA is a more robust effect. It is possible that the observed effect in Rac1 may have some impact on spine remodeling (Christoffel et al, 2011; Golden et al, 2013); however, this may be masked. For example, the RhoA increase in the unpaired mice is nearly 100% more than in the paired mice (178 vs 95%); therefore, it is possible that the larger RhoA increase in the unpaired mice results in the removal of an increased number of thin spines, which is compensated by the Rac1 increase, therefore normalizing the number of spines lost to similar levels between the paired and unpaired mice. In support of the concept that RhoA is more robustly activated by pairing morphine with environmental cues, intra-hippocampal injection of the ROCK inhibitor, which results in a downstream blockade of the RhoA signaling pathway, completely prevented the expression of morphine CPP.
Studies by us and others have demonstrated that the hippocampus has a critical role processing contextual associations with rewards including natural rewards, such as food, and drugs of abuse (Ferbinteanu and McDonald, 2001; Ito et al, 2008; Xia et al, 2011; Fakira et al, 2014). In addition, the hippocampus is known for its role in processing spatial information and memories (Moser et al, 1994; Mahmmoud et al, 2015) via spine remodeling in the dorsal hippocampus. Although these two types of memories and/or associations may be controlled via different mechanisms, they still both require regulation by hippocampal neurons. More recent studies expanding on Seigel’s (Siegel, 1976, 1978; Li et al, 2003; Lisman and Grace, 2005; Sarantis et al, 2012) morphine tolerance studies demonstrate that lesioning the dorsal hippocampus blocks context-dependent morphine tolerance in mice (Huroy et al, 2015) further supporting the notion that the hippocampus is essential in the processing of morphine–context associations. The finding that RhoA signaling is involved in hippocampal functions that process spatial and contextual cues is supported by other studies. In one study, blockade of RhoA in the hippocampus impaired memory retrieval on the Morris water maze, whereas RhoA activation enhanced memory retrieval (Dash et al, 2004). In addition, Rho inhibition in the hippocampus blocked the retrieval of conditioned aversion induced by naloxone-precipitated withdrawal (Haditsch et al, 2009). In our study, the inhibition of the RhoA-ROCK pathway occurred during the training sessions and not during the probe test, therefore preventing the acquisition of the drug–context memory but not the retrieval. In studies by Dash et al (2004), mice were trained on the Morris water maze under baseline conditions and inhibition or enhancement of RhoA was induced only prior to the retrieval test (Dash et al, 2004). When taken together, these studies and ours demonstrate that RhoA activity has a role in both spatial memory acquisition and retrieval.
One remaining question is how the presence of environmental cues is signaled to CA1 hippocampus. Environmental cues induce an increase in dopamine activity in the hippocampus (Li et al, 2003; Lisman and Grace, 2005; Sarantis et al, 2012), which may enhance LTP induction at CA1 synapses. A polysynaptic circuit that has been shown to be critical for this mechanism projects from the subiculum to the nucleus accumbens resulting in disinhibition of the palladium, which will subsequently affect the ventral tegmental area. Ultimately, disinhibition in the ventral tegmental area results in increased dopaminergic output back to the hippocampus (Lisman and Grace, 2005). Furthermore, dopamine influences firing activity of hippocampal cells in response to distal and proximal cues in a novel environment (Tran et al, 2008), supporting the notion that dopamine release in the hippocampus has a role encoding these environmental cues. Moreover, dopamine D1 receptor activation influences RhoA and Rac1 activity (Li et al, 2015), and therefore, pairing morphine conditioning with environmental cues may induce structural plasticity by activation of this signaling pathway.
Overall, our results demonstrate that morphine conditioning using either a paired or unpaired morphine CPP design leads to the decrease in thin spine density in the hippocampus. This structural alteration is accompanied by an increase in synaptic RhoA in the hippocampus. Interestingly, these effects are not observed following the administration of morphine at the home cage suggesting that this spine remodeling is dependent on contextual pairing. In addition, these studies demonstrate that the Rho GTPase signaling cascade is involved in morphine–context associations, as inhibition of this pathway locally in the hippocampus during conditioning training completely prevents the expression of morphine place preference. Our findings will help to elucidate a potential mechanistic link between opioid-induced alterations in structural plasticity with the formation and expression as well as the reinstatement of drug-induced conditioned responses.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Bourne J, Harris KM (2007). Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17: 381–386.
Bourne JN, Harris KM (2008). Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci 31: 47–67.
Bourne JN, Harris KM (2011). Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus 21: 354–373.
Bourne JN, Harris KM (2012). Nanoscale analysis of structural synaptic plasticity. Curr Opin Neurobiol 22: 372–382.
Chen Y, Kramar EA, Chen LY, Babayan AH, Andres AL, Gall CM et al (2013). Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol Psychiatry 18: 485–496.
Christoffel DJ, Golden SA, Russo SJ (2011). Structural and synaptic plasticity in stress-related disorders. Rev Neurosci 22: 535–549.
Compton WM, Volkow ND (2006a). Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend 83 (Suppl 1): S4–S7.
Compton WM, Volkow ND (2006b). Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend 81: 103–107.
Crombag HS, Bossert JM, Koya E, Shaham Y (2008). Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci 363: 3233–3243.
Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S et al (2001). Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry 158: 1680–1686.
Dash PK, Orsi SA, Moody M, Moore AN (2004). A role for hippocampal Rho-ROCK pathway in long-term spatial memory. Biochem Biophys Res Commun 322: 893–898.
Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ et al (2012). Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J Neurosci 32: 6957–6966.
Dumitriu D, Rodriguez A, Morrison JH (2011). High-throughput, detailed, cell-specific neuroanatomy of dendritic spines using microinjection and confocal microscopy. Nat Protoc 6: 1391–1411.
Esmaeili MH, Kermani M, Parvishan A, Haghparast A (2012). Role of D1/D2 dopamine receptors in the CA1 region of the rat hippocampus in the rewarding effects of morphine administered into the ventral tegmental area. Behav Brain Res 231: 111–115.
Fakira AK, Portugal GS, Carusillo B, Melyan Z, Moron JA (2014). Increased small conductance calcium-activated potassium type 2 channel-mediated negative feedback on N-methyl-D-aspartate receptors impairs synaptic plasticity following context-dependent sensitization to morphine. Biol Psychiatry 75: 105–114.
Ferbinteanu J, McDonald RJ (2001). Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus 11: 187–200.
Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K et al (2013). Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med 19: 337–344.
Haditsch U, Leone DP, Farinelli M, Chrostek-Grashoff A, Brakebusch C, Mansuy IM et al (2009). A central role for the small GTPase Rac1 in hippocampal plasticity and spatial learning and memory. Mol Cell Neurosci 41: 409–419.
Harvey CD, Yasuda R, Zhong H, Svoboda K (2008). The spread of Ras activity triggered by activation of a single dendritic spine. Science 321: 136–140.
Hering H, Sheng M (2001). Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci 2: 880–888.
Huroy S, Kanawaty A, Magomedova L, Cummins CL, George SR, van der Kooy D et al (2015). EphB2 reverse signaling regulates learned opiate tolerance via hippocampal function. Behav Brain Res 300: 85–96.
Ikenoya M, Hidaka H, Hosoya T, Suzuki M, Yamamoto N, Sasaki Y (2002). Inhibition of rho-kinase-induced myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in human neuronal cells by H-1152, a novel and specific Rho-kinase inhibitor. J Neurochem 81: 9–16.
Ito R, Robbins TW, Pennartz CM, Everitt BJ (2008). Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci 28: 6950–6959.
Kelsey JE, Aranow JS, Matthews RT (1990). Context-specific morphine withdrawal in rats: duration and effects of clonidine. Behav Neurosci 104: 704–710.
Kobrin KL, Moody O, Arena DT, Moore CF, Heinrichs SC, Kaplan GB (e-pub ahead of print 19 June 2015). Acquisition of morphine conditioned place preference increases the dendritic complexity of nucleus accumbens core neurons. Addict Biol.
Li J, Gu J, Wang B, Xie M, Huang L, Liu Y et al (2015). Activation of dopamine D1 receptors regulates dendritic morphogenesis through Rac1 and RhoA in prefrontal cortex neurons. Mol Neurobiol 51: 1024–1037.
Li S, Cullen WK, Anwyl R, Rowan MJ (2003). Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci 6: 526–531.
Liao D, Grigoriants OO, Wang W, Wiens K, Loh HH, Law PY (2007). Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci 35: 456–469.
Liao D, Lin H, Law PY, Loh HH (2005). Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci USA 102: 1725–1730.
Lisman JE, Grace AA (2005). The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46: 703–713.
Mahmmoud RR, Sase S, Aher YD, Sase A, Groger M, Mokhtar M et al (2015). Spatial and working memory is linked to spine density and mushroom spines. PLoS One 10: e0139739.
Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H (2001). Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci 4: 1086–1092.
McNair K, Spike R, Guilding C, Prendergast GC, Stone TW, Cobb SR et al (2010). A role for RhoB in synaptic plasticity and the regulation of neuronal morphology. J Neurosci 30: 3508–3517.
Miller EC, Zhang L, Dummer BW, Cariveau DR, Loh H, Law PY et al (2012). Differential modulation of drug-induced structural and functional plasticity of dendritic spines. Mol Pharmacol 82: 333–343.
Moron JA, Gullapalli S, Taylor C, Gupta A, Gomes I, Devi LA (2010). Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology 35: 955–966.
Moser MB, Trommald M, Andersen P (1994). An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci USA 91: 12673–12675.
Murakoshi H, Wang H, Yasuda R (2011). Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 472: 100–104.
Nakayama AY, Harms MB, Luo L (2000). Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 20: 5329–5338.
Nakazawa T, Kuriu T, Tezuka T, Umemori H, Okabe S, Yamamoto T (2008). Regulation of dendritic spine morphology by an NMDA receptor-associated Rho GTPase-activating protein, p250GAP. J Neurochem 105: 1384–1393.
Napier TC, Herrold AA, de Wit H (2013). Using conditioned place preference to identify relapse prevention medications. Neurosci Biobehav Rev 37 (9 Pt A): 2081–2086.
Nishiyama J, Yasuda R (2015). Biochemical computation for spine structural plasticity. Neuron 87: 63–75.
O'Brien CP, Childress AR, McLellan AT, Ehrman R (1992). A learning model of addiction. Res Publ Assoc Res Nerv Ment Dis 70: 157–177.
Portugal GS, Al-Hasani R, Fakira AK, Gonzalez-Romero JL, Melyan Z, McCall JG et al (2014). Hippocampal long-term potentiation is disrupted during expression and extinction but is restored after reinstatement of morphine place preference. J Neurosci 34: 527–538.
Robinson TE, Gorny G, Savage VR, Kolb B (2002). Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse 46: 271–279.
Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ (2010). The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci 33: 267–276.
Sarantis K, Antoniou K, Matsokis N, Angelatou F (2012). Exposure to novel environment is characterized by an interaction of D1/NMDA receptors underlined by phosphorylation of the NMDA and AMPA receptor subunits and activation of ERK1/2 signaling, leading to epigenetic changes and gene expression in rat hippocampus. Neurochem Int 60: 55–67.
Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW (2009). Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci 29: 2876–2884.
Siegel S (1976). Morphine analgesic tolerance: its situation specificity supports a Pavlovian conditioning model. Science 193: 323–325.
Siegel S (1978). Tolerance to the hyperthermic effect of morphine in the rat is a learned response. J Comp Physiol Psychol 92: 1137–1149.
Tashiro A, Minden A, Yuste R (2000). Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex 10: 927–938.
Tran AH, Uwano T, Kimura T, Hori E, Katsuki M, Nishijo H et al (2008). Dopamine D1 receptor modulates hippocampal representation plasticity to spatial novelty. J Neurosci 28: 13390–13400.
Wang J, Wang YH, Hou YY, Xi T, Liu Y, Liu JG (2013a). The small GTPase RhoA, but not Rac1, is essential for conditioned aversive memory formation through regulation of actin rearrangements in rat dorsal hippocampus. Acta Pharmacol Sin 34: 811–818.
Wang X, Cahill ME, Werner CT, Christoffel DJ, Golden SA, Xie Z et al (2013b). Kalirin-7 mediates cocaine-induced AMPA receptor and spine plasticity, enabling incentive sensitization. J Neurosci 33: 11012–11022.
Xia Y, Portugal GS, Fakira AK, Melyan Z, Neve R, Lee HT et al (2011). Hippocampal GluA1-containing AMPA receptors mediate context-dependent sensitization to morphine. J Neurosci 31: 16279–16291.
Yagishita S, Hayashi-Takagi A, Ellis-Davies GC, Urakubo H, Ishii S, Kasai H (2014). A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345: 1616–1620.
Yuste R, Bonhoeffer T (2001). Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci 24: 1071–1089.
Zheng H, Zeng Y, Chu J, Kam AY, Loh HH, Law PY (2010). Modulations of NeuroD activity contribute to the differential effects of morphine and fentanyl on dendritic spine stability. J Neurosci 30: 8102–8110.
Acknowledgements
Thanks to Dr Adrianne Wilson-Poe for her comments on the manuscript and Ms. Sarah Satiel for her technical assistance. This work was supported by NIH grant R01 DA027460, R21 DA036826 to JAM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Fakira, A., Massaly, N., Cohensedgh, O. et al. Morphine-Associated Contextual Cues Induce Structural Plasticity in Hippocampal CA1 Pyramidal Neurons. Neuropsychopharmacol 41, 2668–2678 (2016). https://doi.org/10.1038/npp.2016.69
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2016.69
This article is cited by
-
A short period of early life oxytocin treatment rescues social behavior dysfunction via suppression of hippocampal hyperactivity in male mice
Molecular Psychiatry (2022)
-
Formation of a morphine-conditioned place preference does not change the size of evoked potentials in the ventral hippocampus–nucleus accumbens projection
Scientific Reports (2019)