Abstract
We previously reported very low levels of dopamine in post-mortem striatum of chronic methamphetamine users, raising the possibility that restoration of normal dopamine levels could help in this addiction and perhaps prevent early relapse. To establish relevance of this finding to the living brain, we tested whether striatal [11C]-(+)-dihydrotetrabenazine binding, a vesicular monoamine transporter probe sensitive to changes in (stored) vesicular dopamine, is elevated in methamphetamine users. Chronic methamphetamine users underwent [11C]-(+)-dihydrotetrabenazine positron emission tomography scans during early (mean 2.6 days) and later (~10 days) abstinence. Striatal [11C]-(+)-dihydrotetrabenazine binding was elevated (suggesting low stored dopamine) in methamphetamine users (n=28; 2.6 days after last use) relative to controls (n=22) (+28%, p<0.0001) and correlated with severity and recency of drug use and with cognitive impairment and withdrawal symptoms. Mean [11C]-(+)-dihydrotetrabenazine binding levels in the subgroup of methamphetamine users who could remain abstinent ~10 days following last use (n=17) were normal at the follow-up scan. Our imaging data support post-mortem findings and suggest that chronic methamphetamine users have low brain levels of stored dopamine during very early abstinence from MA, which could contribute to behavioral and cognitive deficits. Findings also suggest a rapid recovery of stored dopamine in some methamphetamine users who become abstinent and who therefore might not benefit from dopamine replacement medication (eg, levodopa). Further study is necessary to establish whether those users who could not maintain abstinence for the second scan might have a more severe and persistent dopamine deficiency and who could benefit from this medication.
Similar content being viewed by others
INTRODUCTION
Methamphetamine (MA) and its metabolite amphetamine are widely used psychostimulants used for both ‘recreational’ and therapeutic (eg, attention deficit hyperactivity disorder, obesity) purposes (Kish, 2008). MA stimulates release of the brain neurotransmitter dopamine, considered to be involved in the rewarding property of MA (see Kish, 2008). In two post-mortem brain investigations, we reported very low (up to 90% depletion) levels of dopamine in the striatum (caudate, putamen) of the brain of some recreational MA users who all tested positive for the drug at autopsy (Moszczynska et al, 2004; Wilson et al, 1996a). This suggested that recreational doses of MA might cause a massive release of dopamine in humans sufficient to deplete, at least transiently, tissue stores of dopamine, and, speculatively, that treatment of MA users during drug rehabilitation with dopamine substitution medication (levodopa) might normalize the deficit and perhaps diminish risk for relapse and or behavioral (eg, cognitive and affective) disturbances. Our finding is in line with a preliminary positron emission tomography (PET) [11C]raclopride study in MA users suggesting low synaptic release of striatal dopamine in chronic MA users scanned during abstinence (>2 weeks) and which predicted treatment outcome (ie, relapse) (Wang et al, 2012).
To establish whether our post-mortem brain data might reflect dopamine changes in the living brain, we previously conducted a preliminary PET study in chronic MA users using binding of a radiolabeled vesicular monoamine transporter 1 (VMAT2) probe, [11C]-(+)-dihydrotetrabenazine ((+)[11C]DTBZ) (Koeppe et al, 1996), that, at low concentrations, is sensitive to intraneuronal endogenous dopamine (intravesicular dopamine and (+)[11C]DTBZ compete for the same site on VMAT2) (Boileau et al, 2008, see Tong et al, 2008). In this regard, the use of (+)[11C]DTBZ PET measurement to assess indirectly vesicular dopamine levels departs from the view that VMAT2 density is related to the integrity of substantia nigra dopamine neurons and ‘not subject to drug- or lesion-compensatory regulation’ (Vander Borght et al, 1995). The first hint that striatal (+)[11C]DTBZ binding to VMAT2 might be used to assess differences in stored dopamine came from the unexpected (at the time) finding of de La Fuente-Fernandez et al (2003) who reported increased striatal VMAT2 binding in a small group of patients with dopa-responsive dystonia having a metabolic deficiency of dopamine. Later, human and animal studies showed that binding of a tracer dose of [11C]DTBZ to the striatum could be increased by drugs, including amphetamine, that caused some depletion of intraneuronal stores of dopamine and decreased by agents (eg, levodopa) that increased vesicular dopamine levels (de la Fuente-Fernandez et al, 2009; Kilbourn et al, 2010; Tong et al, 2008). Results of our cross-sectional study supported the notion that some MA users during early abstinence might have a striatal dopamine deficiency, as inferred from elevated (+)[11C]DTBZ binding (Boileau et al, 2008). We now extend this study by using a longitudinal assessment during acute and early, postacute withdrawal to understand better time course, reversibility, and relationship with ‘relapse’ and behavioral function. We have also included a psychostimulant drug control group (cocaine users) and a more representative sample size. Our findings suggest that a transient brain dopamine deficiency occurs in MA users in very early abstinence and is associated with poor(er) cognitive function and a more negative withdrawal state.
MATERIALS AND METHODS
Subjects
Twenty-two healthy subjects (HCs), 28 non-treatment-seeking, active MA users, and nine cocaine users self-referred (in response to web advertisement) to participate in the study. Data from 12 HCs have previously been reported elsewhere (Boileau et al, 2008), whereas all drug users are new recruits. Table 1 provides demographic characteristics. All subjects provided written informed consent approved by the Centre for Addiction and Mental Health Research Ethics Board. Subjects were (1) males or females aged 19–45 years, and had (2) no significant medical conditions/head trauma. HC subjects had (1) no current or personal history of Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV Axis I disorders as per Semi-Structured Clinical Interview (First et al, 1995); (2) no current (12 months) abuse or dependence of drugs of abuse (except nicotine); and (3) negative urinalysis (GC-MS) and scalp hair toxicology (except for cannabis). All MA and cocaine users (1) met the DSM-IV criteria for drug abuse/dependence (for MA and cocaine, respectively) and had (2) positive urinalysis (GC-MS) and scalp hair toxicology for MA in MA users and cocaine in cocaine users. All subjects were compensated for their time for participation in this study.
(+)[11C]DTBZ Image Acquisition Protocol and Analysis
(+)[11C]DTBZ was prepared as described in Boileau et al (2008) and PET images were acquired on a CPS-HRRT neuro-PET camera system (Siemens Medical Imaging).
Non-treatment-seeking drug users (and a subset of HC subjects) were invited to participate in two PET scans during early withdrawal, ~1 week apart. In MA and cocaine users, drug use during the between-scan period was recorded at the second PET session. Drug users who did not remain abstinent (ie, who relapsed as reported by the participants or as evidenced by positive urine) during this period were not scanned.
On scan days, subjects provided urine and blood samples for drug toxicology (urine: BTNX.com multidrug test panel, CAMH Clinical Lab broad-spectrum drug screen; blood: United States Drug Testing Laboratory (USDTL), De Plains, IL), filled out mood, drug withdrawal, and craving questionnaires and completed a battery of cognitive tests (Supplementary Tables 1 and 2). Following a brief transmission scan, the emission scans started with the bolus injection of ~10 mCi of (+)[11C]DTBZ. Emission data were acquired for 60 min in list mode and raw data were reconstructed by Fourier rebinning (FORE) 2D filtered back projection algorithms (Defrise et al, 1997).
Proton-density magnetic resonance imaging were obtained on a Signa 1.5-T scanner (General Electric, Milwaukee, WI) for the purpose of region of interest (ROI) selection. Details of ROI delineation are described in Rusjan et al (2006). In brief, each individual’s set of automatically created ROIs, which included three bilateral subcompartments of the striatum (the associative striatum (AST), limbic striatum (LST), and sensorimotor striatum (SMST)) as described in Martinez et al (2003), was aligned and resliced to match the dimension of the PET images (using SPM8). The occipital cortex was selected as the region of reference, that is, showing only trace levels of VMAT2 protein (Tong et al, 2011). (+)[11C]DTBZ binding to VMAT2 (ie, non-displaceable binding potential; BPND) was estimated in each ROI using the simplified reference tissue method (SRTM; Gunn et al, 1997; Lammertsma and Hume, 1996) and the occipital cortex time–activity curve as an input function (Chan et al, 1999; Koeppe et al, 1996) as implemented in PMOD (version 2.8.5; PMOD Technologies, Zurich, Switzerland). The SRTM has been shown to be an appropriate model for quantifying (+)[11C]DTBZ data in humans without arterial input function (Chan et al, 1999; Koeppe et al, 1996).
Parametric images of (+)[11C]DTBZ binding were generated by estimating SRTM parameters as described in Gunn et al (1997). Each parametric map was spatially normalized to an anatomical template (MNI) using SPM8 normalization and coregistration tools (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Once in the same space, BPND maps were statistically investigated to assess significant contrasts between groups using independent sample t-test analysis (SPM8). Voxel with BPND values <0.2 were excluded from the search. A significance threshold was set as p<0.01 with FWE correction for multiple comparisons.
Statistical Analyses
Comparisons between (+)[11C]DTBZ VMAT2 BPND in the different ROIs in the HCs, MA, and cocaine user groups were conducted by using a repeated-measures ANOVA (SPSS 20) with a within-subject factor (ROI) and a between-subject factor (Group). When appropriate, least significant difference t-tests, Bonferroni-corrected for planned comparison, were applied to determine the significance of regional differences in (+)[11C]DTBZ BPND between groups. Pearson’s product-moment correlation coefficients were used to examine the putative relationships between behavioral and plasma meth/amphetamine measures and regional (+)[11C]DTBZ BPND. A probability value of 0.05 was selected as the significance threshold.
RESULTS
Subjects’ demographic information is reported in Table 1. Twenty-eight MA users, 9 cocaine users, and 22 HCs completed 1 PET scan and 17 MA users, 8 cocaine users, and 9 HCs remained in the study to complete another PET scan ~7 days later. Drug users were matched with HCs with respect to age and gender (cocaine users were marginally older than HCs); however, the level of education and IQ tended to be higher in HCs and the rate of nicotine (and cannabis) smoking, depressive symptomatology were self-reported to be higher in the drug-using groups but not different between MA and cocaine users. Stimulant users also had greater rates of comorbid mood disorders relative to healthy controls. MA and cocaine were the primary and preferred self-reported drugs of abuse in the MA and cocaine users, respectively, and all had used the drug for at least 1 year and were still currently using. MA and cocaine users scored above threshold for optimal discrimination between the presence and absence of a DSM-IV diagnosis of amphetamines dependence (Topp and Mattick, 1997) and cocaine (Kaye and Darke, 2002) and had drug use severity in the intermediate to substantial range based on the Drug Abuse Screening Test (DAST) (Table 2). MA was found in scalp hair of the 28 MA users and cocaine and/or its metabolite benzoylecgonine in hair of the 9 cocaine users, whereas none of these were detected in hair of HCs. Cocaine or metabolite was found in hair of 17 (60%) of the MA users but was only detectable in urine in a single case during the study period. In contrast, cross-use of MA in cocaine users was minimal, with only 1/9 cases providing an MA-positive hair and urine sample. There were no significant differences in (+)[11C]DTBZ scan parameters between groups and scans (Table 1).
PET Results
MA users have greater (+)[11C]DTBZ binding during early abstinence (~2–3 days)
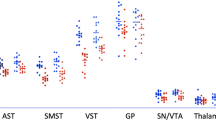
A repeated-measure ANOVA (ROI (3) × Group (3)) investigating differences in (+)[11C]DTBZ binding between HCs and drug-using groups during early abstinence (2.6 days, range 1–7 days) yielded a significant main effect of group (F(2,56)=9.581; p<0.0001) and an ROI × Group interaction (F(1.485, 83.146)=5.077; p=0.001). Pairwise contrast (Bonferroni-corrected) showed that MA use was associated with greater striatal (+)[11C]DTBZ levels when compared with HCs (+28%, p<0.0001) and relative to cocaine users (p=0.038). In contrast, striatal (+)[11C]DTBZ levels in cocaine users were within the normal range (+6%, p=1.0). In MA users, above-normal striatal [(+)[11C]DTBZ levels occurred in the AST (+31%, p<0.0001), LST (+24%, p=0.01), and in the SMST (+26%, p<0.0001); differences from cocaine users were significant in the AST (+22%, p=0.026) and LST (+30%, p=0.027) but not in the SMST (+16%, 0.126) (Table 3; see Figure 1 for the scatter plots). These findings were echoed in our voxel-wise investigation in which we found bilateral clusters of greater (+)[11C]DTBZ binding in the striatum of MA users vs HC (Figure 2). Adjusting ANOVA for group differences in use of cigarettes per day, weekly alcohol consumption, current cannabis use, and self-reported depressive symptoms did not change the main finding of higher (+)[11C]DTBZ binding in MA users overall (vs HCs and cocaine users), respectively, F(2, 55)=5.723; p=0.006, F(2, 43)=5.036; p=0.011, and F(2, 43)=5.723; p=0.006. Interestingly, we noted that MA users who were unable to remain abstinent for the second scan (chose MA over honorarium to compensate for the time spent in the study), and for that reason were not scanned a second time, that is, who relapsed (‘relapsers’, n=7), had 17% (ANCOVA with days of abstinence before PET scan as factor: p=0.035) higher striatal (+)[11C]DTBZ binding at scan 1 compared with those who were able to abstain (‘abstainers’, n=17). Both subgroups of the MA users had significantly higher (+)[11C]DTBZ binding compared with the healthy controls at the first scan, with the exception of the binding in LST of the ‘abstainers’ (Table 3).
Regional [11C]-(+)-dihydrotetrabenazine ((+)[11C]DTBZ) binding at first scan (very early abstinence) for healthy controls, methamphetamine (MA) users, and cocaine users. Note that striatal (+)[11C]DTBZ binding is higher than controls in MA users but not in cocaine users. AST, associative striatum; FS, full striatum; LST, limbic striatum; MA, methamphetamine; SMST, sensorimotor striatum.
T-statistical map overlaid average T1-weighted magnetic resonance imaging (MRI) showing large clusters of significantly greater [11C]-(+)-dihydrotetrabenazine ((+)[11C]DTBZ) binding (lower vesicular dopamine) in the striatum of methamphetamine (MA) users during early abstinence (~2–3 days) relative to healthy controls (left cluster, Ke: 447, PDR-corrected >0.0001, peak voxel in the cluster: t=6.2; right cluster, Ke: 299, PDR-corrected >0.0001, peak voxel in the cluster: t=5.8). Top panel, Montreal Neurological Institute (MNI) coordinates for the Y axis; bottom panel, MNI coordinates for the Z axis.
Rapid recovery of (+)[11C]DTBZ binding in some MA users (after ~10-day abstinence)
Investigating whether striatal (+)[11C]DTBZ binding might change with time, a subset of subjects who remained in the study (MA users: n=17; cocaine users: n=8; HC: n=9) was scanned ~1 week following the first scan (on average 10 days since last drug use). As shown in Table 3 (see Figure 3 for scatter plots), mean striatal binding levels in both cocaine users and MA users measured 1 week following first scan were not significantly different compared with those in HCs (+3% in MA users; +5% in cocaine users vs HCs; F(2, 48)=0.288, p=0.751). In MA users, there was an overall 11% (p=0.055) reduction in striatal (+)[11C]DTBZ binding from the first scan; however, the interindividual variability was high (range: −34% to +29%, 6/17 cases showed increases vs ‘normalization’).
[11C]-(+)-dihydrotetrabenazine ((+)[11C]DTBZ) binding in full striatum at first scan (early abstinence) and second scan (continued abstinence about 1 week later) for healthy controls, methamphetamine (MA) users, and cocaine users. Note that higher than normal binding at first scan returns to control levels at second scan in MA users.
Higher (+)[11C]DTBZ binding in MA users during early abstinence correlated with severity of recent drug use and behavioral/cognitive deficits
Pearson’s product-moment correlations revealed that in MA users (n=28) during early abstinence (2.6 days), severity (frequency) of recent use (full striatum: r=0.68, p<0.0001), and calculated cumulative dose exposure (last month) (full striatum: r=0.48, p=0.009), as well as days of abstinence (AST: r=−0.49, p=0.008) and plasma levels of MA (full striatum: r=0.5; p=0.008) before the first PET scan correlated with (+)[11C]DTBZ binding. In line with the time dependency of MA on (+)[11C]DTBZ binding, we found that MA users who used ~24 h before the scan (n=10, ie, yesterday) had higher (+)[11C]DTBZ binding relative to those who used more than 2 days (n=18, 2–7 days) before the scan. Specifically, this corresponded to a 52, 45, and 45% greater (+)[11C]DTBZ binding in AST, LST, and SMST relative to HCs (and 20, 13, and 15% in AST, LST, and SMST relative to those who used more than 2 days before the scan). Furthermore, (+)[11C]DTBZ binding measured at the first scan predicted measurements at the second scan (AST: r=0.49, p=0.045; LST: r=0.76, p<0.001; full striatum: r=0.54, p=0.026) such that individuals with higher (+)[11C]DTBZ binding during earlier, acute withdrawal (~2 days) had higher (+)[11C]DTBZ binding during later abstinence. Although relative to HCs, MA users did not have an overall marked impairment in cognitive and behavioral function, some slight deficits were noted (see Supplementary Tables 1 and 2).
We investigated whether ‘abnormal’ (+)[11C]DTBZ binding in MA users (n=28, first scan) correlated with behavior: higher striatal (+)[11C]DTBZ binding (presumably low levels of stored dopamine) correlated with cognitive deficits in verbal memory (Hopkins Verbal Learning Task verbal recall: r=−0.43, p=0.03; cued verbal recall: r=−0.46, p=0.02; and verbal recognition: r=−0.46, p=0.01), decision-making (delay discounting, ie, the tendency to choose a smaller sooner reward over a larger later reward as measured by the Delay Discounting Task: r=0.43, p=0.03), and speed of processing (Continuous Performance Task; hit reaction time: r=0.43, p=0.03 in SMST). We also found that self-reported withdrawal symptoms and daytime sleepiness tended to be greater in MA users with higher (+)[11C]DTBZ levels (Amphetamine Withdrawal Questionnaire with LST: r=0.37, p=0.051; Epworth sleepiness scale with whole striatum: r=0.44, p=0.011). In line with the above finding, smaller changes in (+)[11C]DTBZ binding between scan 1 and scan 2 were associated with persistently poorer performance (partial correlation controlling for differences in baseline (+)[11C]DTBZ) in verbal memory (HVLT cued recall with full striatum: r=−0.6, p=0.02) and speed of processing (Continuous Performance Task (omission) and SMST; r=−0.6, p=0.03).
DISCUSSION
Our new PET data confirm and extend our earlier preliminary imaging findings (Boileau et al, 2008) by showing that striatal (+)[11C]DTBZ binding is not decreased but markedly increased in MA users during early abstinence and related to recent severity of drug use—we suggest that this finding reflects a depletion of vesicular dopamine.
The sharp abstinence time-dependent binding drop-off in the cross-sectional analysis (Boileau et al, 2008) is supported by our longitudinal data in a subgroup of MA users (‘abstainers’) who had normal binding levels 1 week (~10 days of abstinence) following the first scan—it is also in line with the time course of MA withdrawal in which some symptoms (dysphoria, anhedonia, fatigue) have been described to peak at 24 h after last use and steadily return to near-normal range within 7–10 days (McGregor et al, 2005; Zorick et al, 2010).
Our findings that MA users who were unable to remain abstinent to complete the second scan (‘relapsers’) and those with poorer cognitive performance and greater withdrawal symptoms had higher (+)[11C]DTBZ binding during acute, early withdrawal and that (+)[11C]DTBZ binding at that time point predicted persistently high (+)[11C]DTBZ binding suggest that low vesicular dopamine levels in early abstinence could increase propensity for relapse. This interpretation is, however, limited by the possibility, for example, that high (+)[11C]DTBZ binding (and the correlations with behaviors) is simply a vulnerability factor or explained by the presence of greater rates of depression symptomatology in the more severe MA using individuals.
Results of our autopsied brain and brain PET investigations of ‘dopamine’ measures are generally similar to the currently reported finding and point to differences in the actions of MA vs cocaine in human brain: MA users having marked differences relative to HC (Moszczynska et al, 2004; Wilson et al, 1996a), whereas cocaine users have, at most, only modest differences (Narendran et al, 2012; Wilson et al, 1996b, see Kish, 2014).
A very low dose of amphetamine did not induce increased striatal (+)[11C]DTBZ binding, measured 2 h after administration, in normal humans (Boileau et al, 2010) such that (+)[11C]DTBZ binding was slightly decreased relative to baseline (by 5%). However, in our ex vivo animal study in which a much higher dose could be given, striatal (+)[11C]DTBZ binding was increased following an acute dose of either amphetamine or a dopamine synthesis inhibitor, which would be expected to lower dopamine levels (Tong et al, 2008). Thus, the simplest explanation for the elevated striatal (+)[11C]DTBZ binding in MA users taking much higher doses of MA (350 mg a day compared with ~30 mg typically given in our PET studies) is that high doses of the drug, a dopamine-releasing agent (Kish, 2014), caused a massive release and loss of intraneuronal dopamine consequently increasing binding ability of (+)[11C]DTBZ, which is subject to a competitive interaction with vesicular dopamine. However, we cannot exclude the possibility that VMAT2 (protein) level itself might also be influenced as part of a compensatory process to changes in dopamine levels or that binding of the tracer to VMAT2 might possibly have been reduced to some extent by competition with residual MA (at scan 1) (Partilla et al, 2006). Our PET imaging findings are consistent with and support our previous post-mortem brain findings of a dopamine loss (putamen −61%; caudate −50%; expressed per control mean) in MA users who had taken the drug within 3 days of death, although the intrastriatal difference in the imaging investigation was only slight (and not statistically significant) in the living brain (AST, +31%; SMST, +26%) (Moszczynska et al, 2004; Wilson et al, 1996a). The lack of change in cocaine users is consistent with a less prominent action of cocaine (vs MA) as a dopamine releaser (see Kish, 2014) and the short half-life of cocaine vs MA. In this regard, our study provides further support for the novel use of (+)[11C]DTBZ in detecting changes in tissue levels of dopamine (de La Fuente-Fernandez et al, 2003) and raises additional caution when interpreting differences in (+)[11C]DTBZ levels as reflecting only a change in VMAT2 protein.
Historically, the primary use of the (+)[11C]DTBZ probe in human striatum has been to assess the integrity of nigrostriatal dopamine neurons (eg, in Parkinson’s disease), as most VMAT2 is localized in the striatum to dopamine storage vesicles in dopamine neurons (see Frey et al, 1996; Nandhagopal et al, 2011; Wilson et al, 1996c). Animal data indicate that a high dose of MA can both damage dopamine neurons and cause loss of striatal VMAT2 (Frey et al, 2001; Seiden and Ricaurte, 1987), although human findings have been equivocal (Johanson et al, 2006; Kitamura et al, 2007; Tong et al, 2014; Wilson et al, 1996a; for a review, see Kish, 2014). In the cocaine literature, dopamine neuron status has generally suggested a slight loss of dopaminergic innervation and/or vesicles in some cocaine users (see above and Kish, 2014). Our finding that striatal (+)[11C]DTBZ binding was not decreased in either the MA or cocaine user groups, as it is in conditions of known dopamine neuron loss (Boileau et al, 2008; Frey et al, 1996; Nandhagopal et al, 2011), could be explained by the fact that any reduction because of the loss of neurons was masked by the acute ‘dopamine-lowering’ effects of the drug that would have increased binding. As such, a longer abstinence time (>2 weeks as in Narendran et al, 2012) might have revealed decreased (+)[11C]DTBZ binding (eg, Johanson et al, 2006). Alternatively, and perhaps more likely, MA and cocaine at the doses used by the subjects of our study, might not have caused toxic loss of either dopamine vesicles or neurons.
Does ‘low brain dopamine’ have any therapeutic relevance? Low striatal dopamine, if sufficiently severe, could induce both motor (Parkinsonism) and non-motor (eg, cognitive, motivational, hedonic status) behavioral problems (Ehringer and Hornykiewicz, 1960; Taylor and Saint-Cyr, 1995). We did find that higher (+)[11C]DTBZ was associated with reduced cognitive function (delayed verbal recall, speed of processing, and discounting rate) and severity of withdrawal and that some MA users with the highest (+)[11C]DTBZ binding were unable to remain abstinent at the second scan session (ie, relapsed). Our finding that high (+)[11C]DTBZ binding is related to impaired cognition is in line with our previous study (Boileau et al, 2008) as well as other published reports in MA users (Johanson et al, 2006; McCann et al, 2008; Volkow et al, 2001b), which have shown a link between dopamine neuron markers (dopamine transporter (DAT)) and deficits in verbal recall and psychomotor function, although the recovery of DAT shown in one study (Volkow et al, 2001a; but see McCann et al, 1998, 2008) was not associated with recovery of cognition. Taken together, the above findings suggest that relapse and behaviors conducive to relapse (ie, impaired cognition, greater withdrawal-associated symptoms) in MA users during abstinence might be related to differences in brain dopamine levels. This finding is in line with the report of Wang et al (2012), to our knowledge the only investigation reporting evidence for a dopamine-related predictor of treatment response, showing a relationship between blunted synaptic dopamine release in the striatum of MA users and clinical outcome. It will however need to be established whether low levels of stored dopamine are, in fact, reflected by equally low concentrations of dopamine at the synapse, which contains only a very small fraction of total ‘tissue’ dopamine. We did not find clear evidence in those subjects who were able to remain abstinent for >7 days that a dopamine deficiency might be present in later abstinence (>10 days); this again is partly in line with the finding from Wang et al (2012), which suggests that MA users able to remain abstinent relative to ‘relapsers‘ may have normal levels of dopamine. This possibility was difficult to address fully in our study as many of the MA users were unable to adhere to the protocol and maintain abstinence for 1 week (seven drop outs due specifically to relapse). However, it is reasonable to suggest that some ‘high’-dose (relapsing) users might have a longer lasting biologically relevant striatal dopamine deficiency. Consistent with this possibility, we found that MA users who were unable to remain abstinent for 7 days had higher (+)[11C]DTBZ binding in the striatum and higher (+)[11C]DTBZ binding at early abstinence predicted persistently higher (+)[11C]DTBZ binding. Although none of the ‘relapsers’ received a second scan, as they could not maintain abstinence, the possibility exists that their VMAT2 binding elevation might have been more severe and prolonged even during later abstinence.
The clinical relevance of our findings is best addressed by a drug intervention study in MA users using levodopa, the most efficacious dopamine substitution medication in the treatment of Parkinson’s disease (Hornykiewicz, 2001; Lees et al, 2014). Normalization of levels of a biologically important neurotransmitter could help normalize behavior. However, a practical safety issue is that, based on animal data, levodopa should probably not be administered at a time when residual MA is present in part because of possible damage to dopamine neurons (Thomas et al, 2009)—a consideration that could limit the use of levodopa in MA users who have a transient dopamine deficiency. We also suggest that, before any efficacy investigation, a safety-tolerability clinical trial in MA users of levodopa in subjects who are in a controlled setting and are free of residual drug might be warranted.
Our main findings suggest that stored dopamine is markedly reduced after recreational use of MA but recovers rapidly in some users able to remain abstinent. Higher (+)[11C]DTBZ binding during early abstinence is associated with cognitive impairment, suggesting that low vesicular dopamine, in some MA users, might be involved in the relapse process.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Boileau I, Houle S, Rusjan PM, Furukawa Y, Wilkins D, Tong J et al (2010). Influence of a low dose of amphetamine on vesicular monoamine transporter binding: a PET (+)[11C]DTBZ study in humans. Synapse 64: 417–420.
Boileau I, Rusjan P, Houle S, Wilkins D, Tong J, Selby P et al (2008). Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: is VMAT2 a stable dopamine neuron biomarker? J Neurosci 28: 9850–9856.
Chan GL, Holden JE, Stoessl AJ, Samii A, Doudet DJ, Dobko T et al (1999). Reproducibility studies with 11C-DTBZ, a monoamine vesicular transporter inhibitor in healthy human subjects. J Nucl Med 40: 283–289.
de La Fuente-Fernandez R, Furtado S, Guttman M, Furukawa Y, Lee CS, Calne DB et al (2003). VMAT2 binding is elevated in dopa-responsive dystonia: visualizing empty vesicles by PET. Synapse 49: 20–28.
de la Fuente-Fernandez R, Sossi V, McCormick S, Schulzer M, Ruth TJ, Stoessl AJ (2009). Visualizing vesicular dopamine dynamics in Parkinson's disease. Synapse 63: 713–716.
Defrise M, Kinahan PE, Townsend DW, Michel C, Sibomana M, Newport DF (1997). Exact and approximate rebinning algorithms for 3-D PET data. IEEE Trans Med Imag 16: 145–158.
Ehringer H, Hornykiewicz O (1960). Verteilung von noradrenalin und dopamin (3-hydroxytyramin) im gehirn des menches und ihr verhalten bei erkrankungen des extrapyramiden systems. Klin Wochenschr 38: 1236–1239.
First MB, Spitzer RL, Gibbon M, Williams JBW (1995). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID/NP) New York: Biometrics Research, New York State Psychiatric Institute; (November 2002).
Frey KA, Koeppe RA, Kilbourn MR (2001). Imaging the vesicular monoamine transporter. Adv Neurol 86: 237–247.
Frey KA, Koeppe RA, Kilbourn MR, Vander Borght TM, Albin RL, Gilman S et al (1996). Presynaptic monoaminergic vesicles in Parkinson's disease and normal aging. Ann Neurol 40: 873–884.
Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ (1997). Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 6: 279–287.
Hornykiewicz O (2001). Dopamine and Parkinson’s disease. A personal view of the past, the present, and the future. Adv Neurol 86: 1–11.
Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J et al (2006). Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology 185: 327–338.
Kalasinsky KS, Hugel J, Kish SJ (2004). Use of MDA (the ‘love drug’) and methamphetamine in Toronto by unsuspecting users of ecstasy (MDMA). J Forensic Sci 49: 1106–1112.
Kaye S, Darke S (2002). Determining a diagnostic cut-off on the Severity of Dependence Scale (SDS) for cocaine dependence. Addiction 97: 727–731.
Kilbourn MR, Butch ER, Desmond T, Sherman P, Harris PE, Frey KA (2010). In vivo [11C]dihydrotetrabenazine binding in rat striatum: sensitivity to dopamine concentrations. Nucl Med Biol 37: 3–8.
Kish SJ (2008). Pharmacologic mechanisms of crystal meth. Cmaj 178: 1679–1682.
Kish SJ (2014) Chapter 08: The Pathology of Methamphetamine Use in the Human Brain From: The Effects of Drug Abuse on the Human Nervous System, edited by Bertha Madras and Michael Kuhar. Elsevier: Amsterdam, The Netherlands.
Kitamura O, Tokunaga I, Gotohda T, Kubo S (2007). Immunohistochemical investigation of dopaminergic terminal markers and caspase-3 activation in the striatum of human methamphetamine users. Int J Legal Med 121: 163–168.
Koeppe RA, Frey KA, Vander Borght TM, Karlamangla A, Jewett DM, Lee LC et al (1996). Kinetic evaluation of [11C]dihydrotetrabenazine by dynamic PET: measurement of vesicular monoamine transporter. J Cereb Blood Flow Metab 16: 1288–1299.
Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. Neuroimage 4 (Part 1): 153–158.
Lees AJ, Tolosa E, Olanow CW (2014). Four pioneers of L-dopa treatment: Arvid Carlsson, Oleh Hornykiewicz, George Cotzias, and Melvin Yahr. Mov Disord 30: 19–36.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y et al (2003). Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23: 285–300.
McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J et al (2008). Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse 62: 91–100.
McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA (1998). Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 18: 8417–8422.
McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM (2005). The nature, time course and severity of methamphetamine withdrawal. Addiction 100: 1320–1329.
Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ et al (2004). Why is parkinsonism not a feature of human methamphetamine users? Brain 127 (Part 2): 363.
Nandhagopal R, Kuramoto L, Schulzer M, Mak E, Cragg J, McKenzie J et al (2011). Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson's disease. Brain 134 (Pt 11): 3290–3298.
Narendran R, Lopresti BJ, Martinez D, Mason NS, Himes M, May MA et al (2012). In vivo evidence for low striatal vesicular monoamine transporter 2 (VMAT2) availability in cocaine abusers. Am J Psychiatry 169: 55–63.
Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB (2006). Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther 319: 237–246.
Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F et al (2006). An automated method for the extraction of regional data from PET images. Psychiatry Res 147: 79–89.
Seiden LS, Ricaurte GA (1987) Neurotoxicity of Methamphetamine and Related Drugs. Raven: New York, NY.
Taylor AE, Saint-Cyr JA (1995). The neuropsychology of Parkinson's disease. Brain Cogn 28: 281–296.
Thomas DM, Francescutti-Verbeem DM, Kuhn DM (2009). Increases in cytoplasmic dopamine compromise the normal resistance of the nucleus accumbens to methamphetamine neurotoxicity. J Neurochem 109: 1745–1755.
Tong J, Boileau I, Furukawa Y, Chang LJ, Wilson AA, Houle S et al (2011). Distribution of vesicular monoamine transporter 2 protein in human brain: implications for brain imaging studies. J Cereb Blood Flow Metab 31: 2065–2075.
Tong J, Fitzmaurice P, Furukawa Y, Schmunk GA, Wickham DJ, Ang LC et al (2014). Is brain gliosis a characteristic of chronic methamphetamine use in the human? Neurobiol Dis 67: 107–118.
Tong J, Wilson AA, Boileau I, Houle S, Kish SJ (2008). Dopamine modulating drugs influence striatal (+)-[11C]DTBZ binding in rats: VMAT2 binding is sensitive to changes in vesicular dopamine concentration. Synapse 62: 873–876.
Topp L, Mattick RP (1997). Validation of the amphetamine dependence syndrome and the SAmDQ. Addiction 92: 151–162.
Vander Borght T, Kilbourn M, Desmond T, Kuhl D, Frey K (1995). The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. Eur J Pharmacol 294: 577–583.
Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M et al (2001a). Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21: 9414–9418.
Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D et al (2001b). Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158: 377–382.
Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D et al (2012). Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 17: 918–925.
Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM et al (1996a). Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2: 699–703.
Wilson JM, Levey AI, Bergeron C, Kalasinsky K, Ang L, Peretti F et al (1996b). Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol 40: 428–439.
Wilson JM, Levey AI, Rajput A, Ang L, Guttman M, Shannak K et al (1996c). Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson’s disease. Neurology 47: 718–726.
Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G et al (2010). Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction 105: 1809–1818.
Acknowledgements
This work was supported by US NIH NIDA DA25096 to SJK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Boileau, I., McCluskey, T., Tong, J. et al. Rapid Recovery of Vesicular Dopamine Levels in Methamphetamine Users in Early Abstinence. Neuropsychopharmacol 41, 1179–1187 (2016). https://doi.org/10.1038/npp.2015.267
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.267
This article is cited by
-
Sex differences in neurobehavioral consequences of methamphetamine exposure in adult mice
Psychopharmacology (2022)
-
Altered patterns of fractional amplitude of low-frequency fluctuation and regional homogeneity in abstinent methamphetamine-dependent users
Scientific Reports (2021)
-
Heightened Dopaminergic Response to Amphetamine at the D3 Dopamine Receptor in Methamphetamine Users
Neuropsychopharmacology (2016)