Abstract
Postpartum depression (PPD) has a prevalence rate of 13% and a similarly high proportion of women report a subclinical state of one or more major depressive episode symptoms. The aim was to investigate whether monoamine oxidase-A (MAO-A) VT, an index of MAO-A density, is increased in the prefrontal and anterior cingulate cortex (PFC and ACC), during PPD or when a PPD spectrum symptom, greater predisposition to crying, is present. MAO-A is an enzyme that increases in density after estrogen decline, and has several functions including creating oxidative stress, influencing apoptosis and monoamine metabolism. Fifty-seven women were recruited including 15 first-onset, antidepressant naive, PPD subjects, 12 postpartum healthy who cry due to sad mood, 15 asymptomatic postpartum healthy women, and 15 healthy women not recently pregnant. Each underwent [11C]-harmine positron emission tomography scanning to measure MAO-A VT. Both PPD and greater predisposition to crying were associated with greater MAO-A VT in the PFC and ACC (multivariate analysis of variance (MANOVA), group effect, F21,135=1.856; p=0.019; mean combined region elevation 21% and 14% in PPD and crying groups, respectively, relative to postpartum asymptomatic). Greater MAO-A VT in the PFC and ACC represents a new biomarker in PPD, and the PPD symptom of predisposition to crying. Novel strategies for preventing PPD (and some PPD symptoms) may be possible by avoiding environmental conditions that elevate MAO-A level and enhancing conditions that normalize MAO-A level. These findings also argue for clinical trials in PPD with the newer, well-tolerated MAO-A inhibitor antidepressants.
Similar content being viewed by others
INTRODUCTION
Depressed mood in postpartum is an important issue. Postpartum depression (PPD), defined as a major depressive episode (MDE) starting within the first year after giving birth, is highly impactful (Wisner et al, 2006), being the most common complication of childbearing with a prevalence rate of 13% (O'Hara and Swain, 1996). In addition, the presence of psychiatric illness is associated with more than a 10-fold risk of suicide during the first postpartum year (Comtois et al, 2008). Furthermore, a similar proportion of new mothers report a subclinical state of one or more MDE symptoms but not the full cluster of symptoms for a MDE, a phenomenon termed minor depression or depressed mood (Cox et al, 1987). While the symptoms of PPD are similar to MDE, the underlying neurobiology cannot be assumed to be completely identical since the antecedents of these two illnesses are different: PPD occurs in postpartum when the hormonal milieu involves changes in estrogen, progesterone, and glucocorticoids. whereas the initiation of non-postpartum MDE is often initiated by a period of elevated stress alone (Paykel et al, 1969; O'Hara and Swain, 1996).
To date, there has never been a neurochemical study, either postmortem or in vivo, of first-onset PPD, that is, the first episode of MDE in the early postpartum period. However, there are several imaging studies of PPD that include subjects with previous MDEs. Changes reported include reduced occipital cortex GABA levels, reduced D2 receptor binding in the ventral striatum in postpartum irrespective of PPD, and reduced 5-HT1A receptor binding in the orbitofrontal, subgenual, and mesiotemporal cortex in a sample of unipolar and bipolar PPD (Epperson et al, 2006; Moses-Kolko et al, 2008, 2012). As with many complex psychiatric illnesses, it is generally believed that a number of neuropathological changes contribute to the symptoms of PPD and it is plausible that some of these markers reflect specific vulnerability to PPD, MDE in general, or both conditions. Animal models of PPD have focused upon aberrant signal transduction, reduced neurogenesis, and abnormalities of GABA receptors (R delta and gamma 2 subunits) (Galea, 2008; Maguire and Mody, 2008; Suda et al, 2008). Even so, several other promising mechanisms in the pathology of mood disorders remain largely unexplored such as pro-oxidant states, markers of pro-apoptotic states, mitochondrial dysfunction, and monoamine metabolism (Shao et al, 2008; Gawryluk et al, 2011; Shelton et al, 2011).
Monoamine Oxidase-A (MAO-A) is an enzyme related to these unexplored mechanisms of abnormality in PPD. MAO-A is primarily located on the outer mitochondrial membrane of glia and monoamine releasing neurons (especially norepinephrine releasing neurons). It influences predisposition towards apoptosis, promotes oxidation, and metabolizes monoamines (Youdim et al, 2006). In brain tissue, levels of MAO-A are highly correlated with MAO-A activity and MAO-A VT, an index of MAO-A density, is measurable in vivo in people using [11C]-harmine positron emission tomography (PET) (Nelson et al, 1979; Saura et al, 1992; Ginovart et al, 2006; Sacher et al, 2010; Bacher et al, 2011). In some low mood states, elevated MAO-A VT and MAO-A density occurs in the prefrontal and anterior cingulate cortex (PFC and ACC) (Meyer et al, 2006; Meyer et al, 2009; Sacher et al, 2010; Bacher et al, 2011; Johnson et al, 2011), however, MAO-A has never been investigated in clinical PPD in humans or in animal models of PPD.
In the present study [11C]-harmine PET is applied to investigate MAO-A VT in PPD and in postpartum, with a focus on the PFC and ACC. It is hypothesized that MAO-A VT will be elevated in these regions during PPD and in a subclinical postpartum group who report increased crying from sadness as compared with an asymptomatic postpartum healthy state and healthy women who are not recently pregnant. The PFC and ACC are selected because these regions influence mood and pessimism, key symptoms for generating MDE (Ressler and Mayberg, 2007; Sharot et al, 2007). The focus of the subclinical postpartum group was increased crying from sadness for several reasons: First, greater MAO-A VT has been reported in the PFC and ACC in humans when this symptom is present on day 5 postpartum (Sacher et al, 2010). Second, this symptom is important in later postpartum months, occurring in ∼20% of women (Evans et al, 2001). Third, this symptom is associated with greater vulnerability to mood induction (Dowlati et al, 2014). Fourth, it is strongly predictive of development of later PPD (Yamashita et al, 2000).
MATERIALS AND METHODS
Participants
Fifteen first-onset, postpartum depressed (PPD) subjects, 12 postpartum healthy subjects who cry due to sad mood but who have never had a MDE, 15 asymptomatic postpartum healthy women, and 15 healthy women not recently pregnant were recruited. The demographics for these groups are shown in Table 1. For each study participant, written consent was obtained after the procedures had been fully explained. The study and recruitment procedures were approved by the Research Ethics Board for Human Subjects at the Centre for Addiction and Mental Health, the University of Toronto. Postpartum subjects were within 18 months since giving birth and PPD subjects had onset of symptoms within the first month since giving birth. The presence of PPD was established using the Structured Clinical Interview for DSM-IV (First et al, 1995) and subsequent consultation by a psychiatrist (JH, JS or JHM). The Hamilton Depression Scale (Hamilton, 1960) was also recorded for all subjects. The PPD subjects who had no other comorbid psychiatric illnesses were antidepressant free and free of psychotropic medication for at least 3 months (one subject had a previous antidepressant trial).
Healthy participants were physically healthy and had no psychiatric history. Women above age 45 or who were in perimenopause or menopause were excluded. The group of 12 postpartum subjects who do not have a MDE but cry due to sad mood was defined by a positive answer to question 9 of the Edinburgh Postnatal Depression Scale, ‘I have been so unhappy, I have been crying’. These subjects otherwise met the same criteria as healthy participants.
All subjects were screened to rule out borderline and antisocial personality disorder using the Structured Clinical Interview for DSM-IV for Axis II disorders (Blais and Norman, 1997). All participants underwent a urine drug screen at screening and on the day of the [11C]-harmine PET scan. Subjects with positive results for other substances at screening or on PET scanning day were excluded. All subjects had not taken over-the-counter medications for at least 1 month prior to scanning and healthy subjects had no history of psychotropic medication use. Subjects were required not to drink tea or coffee on the day of scanning. As this latter criterion is difficult for those who drink a lot of coffee, only those who drink less than three cups of tea/coffee per day were enrolled. Given that some MAO-A inhibitor substances are found in some kinds of alcohol, subjects were required not to drink any alcohol the day before and the day of scanning. Because heavy cigarette smoking can alter available brain MAO-A sites through binding of β-carbolines (Fowler et al, 1996; Bacher et al, 2011) all subjects were non-smoking for longer than 1 year.
Scanning Day Protocol
All subjects had a single [11C]-harmine PET scan. The radioactive half-life of Carbon-11 is 20 min. [11C]-harmine has excellent qualities for measuring MAO-A VT showing high affinity and high selectivity for MAO-A, high brain uptake, full reversibility, a high signal-to-noise ratio, and a lack of brain metabolites in the human brain (reviewed in (Meyer et al, 2009; Sacher et al, 2010; Bacher et al, 2011).
All subjects refrained from breastfeeding for more than 13 half-lives of the radiotracer (>260 min) after injection, a cut-off point similar to that in other investigations of the early postpartum period (Moses-Kolko et al, 2005; Sacher et al, 2010). Radioactivity in breast milk was measured at ∼240 min after injection and was indistinguishable from background activity at the time. A Geiger counter measurement performed at the chest surface in all women at the same time point showed levels similar to background radioactivity.
Image Acquisition
A dose of 370 MBq of intravenous [11C]-harmine was administered as a bolus for each PET scan. An automatic blood sampling system was used to measure arterial blood radioactivity continuously for the first 10 min. Manual samples were obtained at 2.5, 7.5, 15, 20, 30, 45, 60, and ∼90 min post injection. The radioactivity in whole blood and plasma was measured as described previously (Ginovart et al, 2006). Frames were acquired as follows: 15 frames of 1 min, then 15 frames of 5 min. [11C]-harmine was of high radiochemical purity (98.69±1.08%) and high specific activity (1660±1044 mCi/μmol) at the time of injection.
The PET images were obtained using an HRRT PET camera (in-plane resolution; full width at half maximum, 3.1 mm; 207 axial sections of 1.2 mm, Siemens Molecular Imaging, Knoxville, Tennessee) in the manner described previously (Meyer et al, 2009; Sacher et al, 2010; Bacher et al, 2011).
Image Analysis
For the region of interest (ROI) method, each participant underwent magnetic resonance imaging (GE Signa 1.5-T scanner; fast spoiled gradient echo, T1-weighted image; x, y, z voxel dimensions, 0.78, 0.78, and 1.5 mm, GE Medical Systems, Milwaukee, Wisconsin). The ROIs were determined on magnetic resonance images that were coregistered to each summed [11C]-harmine PET image using a mutual information algorithm. Regions of interest were determined using a semi-automated method in which regions on a template MRI are transformed on to the individual MRI, via a series of transformation and deformation parameters that match the template image to the coregistered MRI followed by selection of gray matter voxels within the ROI as described previously (Rusjan et al, 2006; Meyer et al, 2009; Sacher et al, 2010; Bacher et al, 2011). The location of the ROI was verified by visual assessment of the ROI on the coregistered MRI and summated [11C]-harmine PET image.
The ROI selected included those for which abnormal function, neurochemistry, or MAO-A density has been implicated in mood regulation and/or mood disorders (Saura et al, 1992; Meyer et al, 2006; Ressler and Mayberg, 2007; Meyer et al, 2009; Price and Drevets, 2010; Sacher et al, 2010; Bacher et al, 2011). The ROIs sampled whole PFC, ACC (Brodmann areas 24, part of 32), putamen, ventral striatum, thalamus, midbrain, and hippocampus.
MAO-A VT can be measured with [11C]-harmine PET. It represents the total tissue binding of [11C]-harmine at equilibrium, of which 85% has specific binding to MAO-A. Hence changes in MAO-A VT may be interpreted as representing changes in harmine binding to MAO-A. The VT can be expressed in terms of kinetic rate parameters as follows VT=(K1/k2) × (k3/k4)+(K1/k2) where K1 and k2 are influx and efflux rate constants for radiotracer passage across the blood brain barrier and k3 and k4 describe the radioligand transfer between the free and non-specific compartment and the specific binding compartment. K1/k2 is similar among different individuals (for further details see Ginovart et al (Ginovart et al, 2006)). For [11C]-harmine PET, VT may be validly and reliably measured with either an unconstrained two-tissue compartment model or with the Logan model with arterial sampling (for which the underestimate of VT is negligible for time activity curves from ROI) (Ginovart et al, 2006), and the latter was applied in this study. This method has been described in greater detail previously (Ginovart et al, 2006; Meyer et al, 2009).
Statistical Analysis
There were two main analyses: the first main analysis was a MANOVA applied to determine the effect of group (PPD, postpartum with crying, postpartum asymptomatic, healthy not recently pregnant) upon MAO-A VT in the two primary ROI, the PFC and ACC; additional comparisons were carried out using the protected Least Significant Difference (LSD) procedure.
The second analysis was a MANOVA to assess the effect of group (PPD, postpartum crying, postpartum asymptomatic, and healthy not recently pregnant) throughout all the brain regions assayed, which included regions that have been implicated in mood disorders and/or have reasonably high concentrations of MAO-A, such as the ventral striatum, dorsal putamen, hippocampus, thalamus, and midbrain.
RESULTS
As shown in the demographics (Table 1), there were no significant differences between groups in age (ANOVA, F3,53=0.031, p=0.993) nor phase of menstrual cycle (χ2(9, N=57)=9.75, p=0.371) at the time of scanning. The first main analysis prioritized the PFC and ACC and showed a significant elevation of MAO-A VT with PPD and postpartum with crying due to sad mood (MANOVA, effect of group, F6,104=3.540, p=0.003), as well as significant main effects in the PFC (F3,53=7.518, p=0.000) and the ACC (F3,53=4.869, p=0.005). Post-hoc Fisher’s LSD tests for the PFC indicate that the mean scores for the PPD (M=23.54, SD=3.45) and the postpartum group with crying (M=22.75, SD=2.93) were significantly different than the mean scores of both the postpartum asymptomatic group (M=19.78, SD=3.62) and the healthy not recently pregnant group (M=19.90, SD=2.14). MAO-A VT in the PFC was elevated by 22% in the MDD group, and 15% in postpartum group with crying, in comparison to the postpartum asymptomatic group. Post-hoc LSD tests for the ACC also indicate that the mean scores for the PPD group (M=24.16, SD=3.85) were significantly different than the mean scores from both the postpartum asymptomatic group (M=20.73, SD=3.74) and the healthy not recently pregnant group (M=20.90, SD=2.42), and that the mean score for postpartum group with crying (M=23.42, SD=2.81) was higher than the postpartum asymptomatic group (M=20.73, SD=3.74). MAO-A VT in the ACC was elevated by 19% in the MDD group, and 13% in postpartum with crying, in comparison to the postpartum asymptomatic group. The mean Edinburgh Postnatal Depression Scale (Cox et al, 1987) in the PPD group was 15.13±4.17.
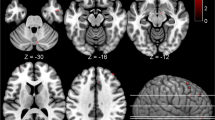
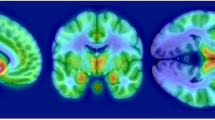
In the analysis of the larger set of regions, MAO-A VT was also significantly elevated in the PPD group and the postpartum group with crying (MANOVA, effect of group, F6,104=3.265, p=0.006) (see Figure 1). Significant univariate effects were seen in five of seven brain regions under investigation, including the PFC, ACC, thalamus, dorsal putamen and midbrain, with a trend towards significance in the ventral striatum (p=0.066) and the hippocampus (p=0.073). There were no significant differences in radioligand binding to plasma protein among groups (53 samples out of 57 subjects available, ANOVA, effect of group, F(3,49)=0.36, p=0.78). The plasma free fraction means for the groups were as follows: 1.26% (±0.59) for healthy not recently pregnant, 1.24% (±0.46) for postpartum asymoptomatic, 1.38% (±0.67) for healthy postpartum with crying, and 1.16% (±0.28) for PPD. Given these results, there is no reason to think that the free fraction accounts for the finding.
Greater monoamine oxidase-A VT level in postpartum depression compared with healthy controls. MAO-A VT was significantly elevated in the postpartum depression (PPD) group and the postpartum group with crying due to sad mood (multivariate analysis of variance, effect of group, F6,104=3.265, p=0.006) and significant univariate effects found in the prefrontal and anterior cingulate cortex (PFC and ACC), thalamus, dorsal putamen, and midbrain (p=0.000–0.035). Post-hoc testing in PFC and ACC showed significant elevation in MAO-A VT in PPD group relative to both healthy groups (p⩽0.05, Least Significant Difference (LSD) test). Postpartum subjects who were crying due to sad mood showed a significant elevation in MAO-A VT relative to both healthy groups in the PFC, and relative to the healthy recently pregnant group in the ACC (p⩽0.05, LSD test).
Additional Exploratory Analyses of Predictors of MAO-A VT in the PFC and ACC
First, a MANOVA was performed after collapsing two postpartum groups (healthy postpartum asymptomatic and otherwise healthy postpartum women who cry due to sad mood) such that three groups remained: healthy not recently pregnant (n=15), healthy postpartum (n=27), and PPD (n=15). Significant main effects were found in MAO-A VT in the PFC (F2,54=7.07, p=0.002) and ACC (F2,54=4.74, p=0.013). Corrected post-hoc tests demonstrated increased MAO-A VT in the PPD group relative to the healthy not recently pregnant group (p=0.003 in PFC and 0.021 in ACC) and relative to the collapsed, healthy postpartum group (p=0.007 in PFC and 0.032 in ACC).
Second, a MANOVA was performed after collapsing the four original groups into two groups: symptomatic (n=27) and asymptomatic (n=30). The symptomatic group was formed by merging healthy postpartum women with crying and PPD women. The asymptomatic group merged healthy not recently pregnant women and postpartum asymptomatic women. MAO-A VT was significantly elevated in the symptomatic group relative to the asymptomatic group (MANOVA, effect of group, F1,55=20.28, p<0.001 in PFC and F1,55=13.29, p=0.001 in ACC).
DISCUSSION
This is the first study to investigate brain MAO-A in PPD and we found that MAO-A VT was highly elevated, particularly in the PFC and ACC in PPD and postpartum women who cry (but are otherwise healthy). In these groups, MAO-A VT also tended to be elevated in other brain regions that are either implicated in the pathophysiology of mood disorders or which have a reasonably high MAO-A density such as the hippocampus, striatum, thalamus, and midbrain. These findings have important implications for understanding the pathophysiology of PPD/PPD spectrum symptoms, creating novel prevention strategies against PPD, and developing specific treatment approaches.
Given the functions of MAO-A, the present study argues that several types of processes that contribute to pathological mood are enhanced in PPD. MAO-A VT is an index of MAO-A level (Tong et al, 2013; Ginovart et al, 2006) and the changes in latter are strongly implicated in the effects of hormonal influences on the MAO-A activity(Nelson et al, 1979; Edelstein and Breakefield, 1986; Saura et al, 1992; Ma et al, 1993; Smith et al, 2004). MAO-A metabolizes monoamines such as serotonin, norepinephrine, and dopamine, creates reactive oxygen species such as hydrogen peroxide (H2O2), thereby promoting a pro-oxidant state (Youdim et al, 2006), suggesting that enhanced monoamine metabolism and a pro-oxidant state may be present during PPD. In addition, greater MAO-A activity is associated with apoptosis-inducing conditions (Ou et al, 2006; Youdim et al, 2006). Increased expression of genes associated with greater apoptotic stress, and increased levels of pro-apoptotic transcription factors in the PFC are associated with MDE although these have not been investigated in PPD (Johnson et al, 2011; Shelton et al, 2011). Thus, the elevation in MAO-A VT in the PFC and ACC in PPD implicates several processes also associated with dysregulated mood, including greater monoamine removal, a pro-oxidative state, and greater predisposition to apoptosis.
Our findings have major implications for developing novel prevention strategies for PPD. Substantial estrogen decline, such as the decline in estrogens over the first few days postpartum consequent to loss of placenta, is normally associated with a very strong, temporary rise in MAO-A VT density and activity (Chevillard et al, 1981; Ma et al, 1993; Smith et al, 2004; Sacher et al, 2010). The present study suggests that MAO-A VT subsequently declines either to normal levels, or does not decline to normal levels, leading to three subsequent outcomes: With a full decline to normative MAO-A VT level in the PFC and ACC, a healthy mood is a likely outcome. With an inadequate decline, and ongoing elevated MAO-A VT levels in the PFC and ACC, either PPD or a tendency to cry due to depressed mood is likely. Not having a full PPD despite the presence of elevated MAO-A VT in the PFC and ACC may reflect greater resiliency against elevated MAO-A levels or against PPD due to a lesser presence of the other markers associated with PPD (such as reduced GABA levels, GABA receptor abnormalities, reduced 5-HT1A binding, reduced neurogenesis etc (Epperson et al, 2006; Galea et al, 2008; Maguire and Mody, 2008; Moses-Kolko et al, 2008; Suda et al, 2008).
Based upon this model, promoting normalization of MAO-A levels after the immediate postpartum period might reduce the probability of PPD and/or the subsyndromal symptom of crying due to sad mood. At a practical level, since chronic stress, heavy cigarette smoking, and pro-apoptotic states facilitate greater MAO-A expression (Filipenko et al, 2002; Ou et al, 2006; Ou et al, 2006a; Bacher et al, 2011), avoidance of these would be expected to be beneficial. Consistent with this, factors related to chronic stress such as perceived reduced partner support are often associated with greater risk for PPD (Brugha et al, 1998; Adewuya et al, 2005). While it may not always be feasible to avoid chronic high stress, it is possible to reduce cigarette smoking during pregnancy (Hauge et al, 2011). Prospective evaluation of depression symptoms during pregnancy and postpartum reported greater severity in women who smoke more heavily, and it is heavy smoking that is associated with greater MAO-A levels (Ludman et al, 2000; Munafo et al, 2008; Bacher et al, 2011). Investigations into mechanisms that increase MAO-A levels and activity are a relatively new direction so this list is anticipated to grow substantially over the upcoming decade. Given the lack of standardized recommendations for preventing PPD, translation of biological models to facilitate normalizing MAO-A level into prevention strategies represents a novel and potentially important direction for long term impact.
To date, randomized double blind clinical trials have largely focused upon selective serotonin reuptake inhibitor treatment for PPD (di Scalea and Wisner, 2009), whereas randomized double blind clinical investigations with MAO-A inhibitors have not yet been conducted. Ideally, treatments should be matched to illness, and SSRI, which raise extracellular serotonin, can be viewed as only a partial match to the pathology of MAO-A levels insofar as there is a match with the MAO-A function of metabolizing serotonin, but a mismatch with the other functions of MAO-A to remove monoamines and promote a pro-oxidative state. Our data would argue for clinical trials of MAO-A inhibitors for treatment of PPD, a feasible strategy with the development of new, well-tolerated MAO-A inhibitors that either rapidly wash out of the periphery or which have high brain to periphery concentrations (Haefely et al, 1992; Youdim et al, 2006).
Some clarifications regarding measurement and interpretation apply to our study. Being a PET neuroimaging study, we chose to measure MAO-A VT because it is computationally efficient, highly stable, and the least variable measure of [11C]-harmine binding. However, since ∼15% of this measure reflects free and non-specific binding (Ginovart et al, 2006) it is assumed that free and non-specific binding do not differ enormously (ie, by more than twofold), between conditions. Finally, the specific binding of [11C]-harmine represents both MAO-A density and affinity since an increase in either could increase [11C]-harmine binding. A change in affinity is unlikely since affinity is similar across brain regions (Bottlaender et al, 2010), and, to the best of our knowledge, MAO-A affinity has not been reported to be modifiable in brain tissue. However, even if affinity of MAO-A alone were increased, this would not necessarily change the functional interpretation of our data as greater affinity of MAO-A for substrate could still represent a mechanism for increased monoaminergic loss and excessive oxidation.
In summary, this is the first neurochemical study of first-onset PPD in humans, and we found significantly greater MAO-A VT, an index of MAO-A density, in the PFC and ACC. These results implicate specific pathological conditions in PPD associated with greater MAO-A levels such as greater monoamine lowering, pro-oxidant state, and greater vulnerability to apoptosis (Ou et al, 2006; Youdim et al, 2006). We also found elevated MAO-A VT in women who experience pronounced crying due to sad mood in postpartum but are otherwise healthy, which suggests that persistent elevation of MAO-A VT in the PFC and ACC is linked to intermittent expression of overly depressed mood. These results suggest a future direction of developing strategies to prevent PPD through avoidance of environmental factors known to raise MAO-A levels or activity, which presently include chronic stress and heavy cigarette smoking, (Filipenko et al, 2002; Ou et al, 2006; Ou et al, 2006a; Youdim et al, 2006; Bacher et al, 2011) although it is anticipated that additional factors will be identified. In addition, the results argue, based upon increased target expression of MAO-A, that randomized double blind clinical trials of PPD treatment should evaluate the MAO-A inhibitor class of antidepressants.
FUNDING AND DISCLOSURE
Dr Meyer is developing (and patenting) natural health products to treat high MAO-A states. Dr Meyer is applying for patents to apply measures of MAO to diagnose or treat mood disorders. It is likely that companies which make medications that affect monoamine receptors or monoamine oxidase binding will seek collaborations with these investigators in the future. Dr Stewart served on the Duloxetine Pregnancy Registry Scientific Advisory Board 2011–present, received one-time Ranbaxy Travel Support in 2012, and is an author on the fetal effects of SSRI’s and the Treatment of Depression in Pregnancy for the publication, ‘UpToDate.’ The remaining authors declare no conflict of interest.
References
Adewuya AO, Fatoye FO, Ola BA, Ijaodola OR, Ibigbami SM (2005). Sociodemographic and obstetric risk factors for postpartum depressive symptoms in Nigerian women. J Psychiatr Pract 11: 353–358.
Bacher I, Houle S, Xu X, Zawertailo L, Soliman A, Wilson AA et al (2011). Monoamine oxidase a binding in the prefrontal and anterior cingulate cortices during acute withdrawal from heavy cigarette smoking. Arch Gen Psychiatry 68: 817–826.
Blais MA, Norman DK (1997). A psychometric evaluation of the DSM-IV personality disorder criteria. J Pers Disord 11: 168–176.
Bottlaender M, Valette H, Delforge J, Saba W, Guenther I, Curet O et al (2010). In vivo quantification of monoamine oxidase A in baboon brain: a PET study using [(11)C]befloxatone and the multi-injection approach. J Cereb Blood Flow Metab 30: 792–800.
Brugha TS, Sharp HM, Cooper SA, Weisender C, Britto D, Shinkwin R et al (1998). The Leicester 500 Project. Social support and the development of postnatal depressive symptoms, a prospective cohort survey. Psychol Med 28: 63–79.
Chevillard C, Barden N, Saavedra JM (1981). Estradiol treatment decreases type A and increases type B monoamine oxidase in specific brain stem areas and cerebellum of ovariectomized rats. Brain Res 222: 177–181.
Comtois KA, Schiff MA, Grossman DC (2008). Psychiatric risk factors associated with postpartum suicide attempt in Washington State, 1992-2001. Am J Obstet Gynecol 199: 120 e121–120 e125.
Cox JL, Holden JM, Sagovsky R (1987). Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 150: 782–786.
di Scalea TL, Wisner KL (2009). Pharmacotherapy of postpartum depression. Expert Opin Pharmacother 10: 2593–2607.
Dowlati Y, Segal ZV, Ravindran AV, Steiner M, Stewart DE, Meyer JH (2014). Effect of dysfunctional attitudes and postpartum state on vulnerability to depressed mood. J Affect Disord 161: 16–20.
Edelstein SB, Breakefield XO (1986). Monoamine oxidases A and B are differentially regulated by glucocorticoids and ‘aging’ in human skin fibroblasts. Cell Mol Neurobiol 6: 121–150.
Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH et al (2006). Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl) 186: 425–433.
Evans J, Heron J, Francomb H, Oke S, Golding J (2001). Cohort study of depressed mood during pregnancy and after childbirth. BMJ 323: 257–260.
Filipenko ML, Beilina AG, Alekseyenko OV, Dolgov VV, Kudryavtseva NN (2002). Increase in expression of brain serotonin transporter and monoamine oxidase a genes induced by repeated experience of social defeats in male mice. Biochemistry (Mosc) 67: 451–455.
First M, Spitzer R, Williams J, Gibbon M (1995). ‘Structured clinical interview for DSM-IV axis I disorders, patient edition (SCID-P), version 2’. Biometrics Research New York, NY, USA.
Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C et al (1996). Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci USA 93: 14065–14069.
Galea LA (2008). Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev 57: 332–341.
Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL et al (2008). Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol 62: 247–260.
Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT (2011). Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14: 123–130.
Ginovart N, Meyer JH, Boovariwala A, Hussey D, Rabiner EA, Houle S et al (2006). Positron emission tomography quantification of [11C]-harmine binding to monoamine oxidase-A in the human brain. J Cereb Blood Flow Metab 26: 330–344.
Haefely W, Burkard WP, Cesura AM, Kettler R, Lorez HP, Martin JR et al (1992). Biochemistry and pharmacology of moclobemide, a prototype RIMA. Psychopharmacology (Berl) 106 (Suppl): S6–14.
Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62.
Hauge L, Toergersen L, Vollrath M (2011). associations between maternal stress and smoking: findings from a population based prospective cohort study. Addiction 107: 1168–1173.
Johnson S, Stockmeier CA, Meyer JH, Austin MC, Albert PR, Wang J et al (2011). The reduction of r1, a novel repressor protein for monoamine oxidase a, in major depressive disorder. Neuropsychopharmacology 36: 2139–2148.
Ludman EJ, McBride CM, Nelson JC, Curry SJ, Grothaus LC, Lando HA et al (2000). Stress, depressive symptoms, and smoking cessation among pregnant women. Health Psychol 19: 21–27.
Ma ZQ, Bondiolotti GP, Olasmaa M, Violani E, Patrone C, Picotti GB et al (1993). Estrogen modulation of catecholamine synthesis and monoamine oxidase A activity in the human neuroblastoma cell line SK-ER3. J Steroid Biochem Mol Biol 47: 207–211.
Maguire J, Mody I (2008). GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron 59: 207–213.
Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A et al (2006). Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry 63: 1209–1216.
Meyer JH, Wilson AA, Sagrati S, Miler L, Rusjan P, Bloomfield PM et al (2009). Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch Gen Psychiatry 66: 1304–1312.
Moses-Kolko E, Wisner K, Berga S, Grace A, Price JC, Mathis C et al (2005). [C-11]raclopride-PET measurement of CNS D2 receptor binding is altered in postpartum depressed women. Biol Psychiatry 57: 34S.
Moses-Kolko EL, Price JC, Wisner KL, Hanusa BH, Meltzer CC, Berga SL et al (2012). Postpartum and depression status are associated with lower [(11)C]raclopride BP(ND) in reproductive-age women. Neuropsychopharmacology 37: 1422–1432.
Moses-Kolko EL, Wisner KL, Price JC, Berga SL, Drevets WC, Hanusa BH et al (2008). Serotonin 1A receptor reductions in postpartum depression: a positron emission tomography study. Fertil Steril 89: 685–692.
Munafo MR, Hitsman B, Rende R, Metcalfe C, Niaura R (2008). Effects of progression to cigarette smoking on depressed mood in adolescents: evidence from the National Longitudinal Study of Adolescent Health. Addiction 103: 162–171.
Nelson DL, Herbet A, Petillot Y, Pichat L, Glowinski J, Hamon M (1979). [3H]Harmaline as a specific ligand of MAO A—I. Properties of the active site of MAO A from rat and bovine brains. J Neurochem 32: 1817–1827.
O'Hara MW, Swain A (1996). Rates and risk of postpartum depression—a meta analysis. Int Rev Psychiatry 8: 37–54.
Ou XM, Chen K, Shih JC (2006). Glucocorticoid and androgen activation of monoamine oxidase A is regulated differently by R1 and Sp1. J Biol Chem 281: 21512–21525.
Ou XM, Chen K, Shih JC (2006a). Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci USA 103: 10923–10928.
Paykel ES, Myers JK, Dienelt MN, Klerman GL, Lindenthal JJ, Pepper MP (1969). Life events and depression. A controlled study. Arch Gen Psychiatry 21: 753–760.
Price J, Drevets W (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology 35: 192–216.
Ressler KJ, Mayberg HS (2007). Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10: 1116–1124.
Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F et al (2006). An automated method for the extraction of regional data from PET images. Psychiatry Res 147: 79–89.
Sacher J, Wilson A, Houle S, Hassan S, Rusjan P, Bloomfield P et al (2010). Elevated brain monoamine oxidase a binding in early postpartum. Arch Gen Psychiatry 67: 468–474.
Saura J, Kettler R, Da Prada M, Richards JG (1992). Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19- 6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci 12: 1977–1999.
Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM et al (2008). Mitochondrial involvement in psychiatric disorders. Ann Med 40: 281–295.
Sharot T, Riccardi AM, Raio CM, Phelps EA (2007). Neural mechanisms mediating optimism bias. Nature 450: 102–105.
Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA et al (2011). Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry 16: 751–762.
Smith LJ, Henderson JA, Abell CW, Bethea CL (2004). Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology 29: 2035–2045.
Suda S, Segi-Nishida E, Newton SS, Duman RS (2008). A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol Psychiatry 64: 311–319.
Tong J, Meyer JH, Furukawa Y, Boileau I, Chang LJ, Wilson AA et al (2013). Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies. J Cereb Blood Flow Metab 33: 863–871.
Wisner KL, Chambers CH, Sit DK (2006). Postpartum depression: A major public health problem. JAMA 296: 2616–2618.
Yamashita H, Yoshida K, Nakano H, Tashiro N (2000). Postnatal depression in Japanese women. Detecting the early onset of postnatal depression by closely monitoring the postpartum mood. J Affect Disord 58: 145–154.
Youdim MB, Edmondson D, Tipton KF (2006). The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7: 295–309.
Acknowledgements
We thank Dr Alexandra Soliman, research co-ordinators Laura Miler and Cynthia Xu, administrative assistant Natasha Bennett, technicians Alvina Ng and Laura Nguyen, chemistry staff Jun Parkes, Armando Garcia, Winston Stableford and Min Wong, and engineers Terry Bell and Ted Harris-Brandts for their assistance with this project. This research received project support from the Canadian Institutes of Health Research (CIHR), the National Alliance for Research on Schizophrenia and Depression (NARSAD), salary support from Canadian Institutes of Health Research (CIHR), the Funds for the Advancement of Scientific Research Austria (FWF), the Society in Science (SiS) Branco Weiss Fellowship, Alexander von Humboldt Foundation, and infrastructure support from the Canadian Foundation for Innovation (CFI), and the Ontario Ministry for Research and Innovation. This research was presented in part at the 2011 Society of Biological Psychiatry and the American College of Neuropsychopharmacology meetings. Drs Meyer, Wilson, and Houle have received operating grant funding for other studies from Eli-Lilly, GlaxoSmithKline, Bristol Myers Squibb, Lundbeck, and SK Life Sciences in the past 5 years and Dr Meyer has consulted to several of these companies, as well as Sepracor, Mylan, and Teva. None of these companies participated in the funding, design or execution of this study, or writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Sacher, J., Rekkas, P., Wilson, A. et al. Relationship of Monoamine Oxidase-A Distribution Volume to Postpartum Depression and Postpartum Crying. Neuropsychopharmacol 40, 429–435 (2015). https://doi.org/10.1038/npp.2014.190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.190
This article is cited by
-
Postpartum depression in Vietnam: a scoping review of symptoms, consequences, and management
BMC Women's Health (2023)
-
The maternal reward system in postpartum depression
Archives of Women's Mental Health (2019)
-
Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders
Psychopharmacology (2019)
-
Postpartum psychiatric disorders
Nature Reviews Disease Primers (2018)
-
Hormonal Cycle and Contraceptive Effects on Amygdala and Salience Resting-State Networks in Women with Previous Affective Side Effects on the Pill
Neuropsychopharmacology (2018)