Abstract
Low doses of psychostimulants, including methylphenidate (MPH), are highly effective in the treatment of attention-deficit/hyperactivity disorder (ADHD). At these doses, psychostimulants improve prefrontal cortex (PFC)-dependent function. Recent evidence indicates that low and clinically relevant doses of psychostimulants target norepinephrine (NE) and dopamine (DA) signaling preferentially in the PFC. To better understand the neural mechanisms responsible for the regional selectivity of low-dose psychostimulant action, it is important to first identify the underlying neurocircuitry. The current study used reverse microdialysis to test the hypothesis that the preferential targeting of PFC catecholamines by low-dose psychostimulants involves direct action within the PFC, reflecting an intrinsic property of this region. For these studies, the effects of varying concentrations of MPH (0.25, 1.0, and 4.0 μM) on NE and DA efflux were examined within the PFC and select subcortical fields in unanesthetized rats. Low concentrations of MPH elicited significantly larger increases in extracellular levels of NE and DA in the PFC than in subcortical regions linked to motor-activating and arousal-promoting actions of psychostimulants (nucleus accumbens and medial septal area, respectively). The differential action of MPH across regions disappeared at higher concentrations. The enhanced sensitivity of PFC catecholamines to low and clinically relevant doses of psychostimulants, at least in part, reflects a unique sensitivity of this region to NE/DA transporter blockade. Available evidence suggests that the increased sensitivity of PFC catecholamines likely involves DA clearance through the NE transporter within the PFC.
Similar content being viewed by others
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is conservatively estimated to affect 3–5% of children and adults (Solanto, 2001; Spencer et al, 2004). Pharmacological treatment is highly effective in the treatment of this disorder, with low-dose psychostimulants currently the most effective and most widely used treatment for ADHD (Greenhill, 2001). In ADHD patients, clinically relevant doses of psychostimulants and other drugs used in the treatment of ADHD improve prefrontal cortex (PFC)-dependent behavioral and cognitive processes, including behavioral inhibition, working memory, and planning (Berridge and Devilbiss, 2011), consistent with evidence implicating the PFC in ADHD (Castellanos and Tannock, 2002). Moreover, these actions of psychostimulants are not unique to ADHD, with similar cognition-enhancing effects observed in healthy human and animal subjects administered clinically relevant doses (Berridge et al, 2006; Gamo et al, 2010; Rapoport and Inoff-Germain, 2002).
Neurochemically, low and clinically relevant doses of the psychostimulant, methylphenidate (MPH; Ritalin), elevate norepinephrine (NE), and dopamine (DA) signaling preferentially within the PFC. Thus, in rats, doses of MPH that produce clinically relevant plasma concentrations and enhance PFC-dependent working memory and sustained attention, elicit larger increases in extracellular NE and DA in the PFC relative to other cortical and subcortical regions (Berridge et al, 2006; Kuczenski and Segal, 2001). This regional selectivity is in contrast to the robust and widespread increases seen with higher doses of psychostimulants (Kuczenski et al, 1997). Consistent with these observations, recent studies demonstrate that when infused directly into the dorsomedial PFC, MPH improves performance in a working memory task, similar to that seen with systemic administration of MPH (Spencer et al, 2012). Combined, these observations indicate that the cognition-enhancing and therapeutic actions of psychostimulants involve, at least in part, preferential targeting of PFC catecholamines.
To date, the mechanisms responsible for the preferential sensitivity of PFC catecholamines to low and clinically relevant doses of psychostimulants are unknown, representing a significant gap in our understanding of both the pharmacology of ADHD and the neurobiology of the PFC. The enhanced sensitivity of PFC catecholamines to low-dose psychostimulants could reflect drug action outside the PFC, including on non-catecholamine PFC-projecting neurons known to modulate catecholamine release (eg, amino acids; Bonanno et al, 1989; Galli et al, 1991). Alternatively, this could reflect local action within the PFC and a unique sensitivity of PFC catecholamines to low concentrations of psychostimulants. For example, in the PFC the NE transporter (NET) has a prominent role in DA clearance from the extracellular space (Carboni et al, 2006, 1990; Moron et al, 2002; Valentini et al, 2004). Evidence suggests that NE and DA binding to the NET is competitive, with increases in either NE or DA within the PFC elevating extracellular levels of the other transmitter (Bymaster et al, 2002; Schmeichel et al, 2012; Valentini et al, 2004). Such a feed-forward process could contribute to the enhanced sensitivity of PFC catecholamines to low-dose psychostimulants.
A critical first step in identifying the mechanism(s) responsible for the preferential targeting of PFC catecholamines by low-dose psychostimulants is the identification of the underlying neurocircuitry. The current studies examined whether the enhanced sensitivity of PFC catecholamines involves direct action of psychostimulants within the PFC. Using a reverse microdialysis approach, we examined the effects of local perfusion of MPH on extracellular levels of NE and DA within the PFC, nucleus accumbens (NAc) and the medial septal area (MSA). These three regions were of particular interest as earlier studies characterized the effects of low and clinically relevant doses of systemically administered MPH on extracellular catecholamines within these regions (Berridge et al, 2006). Results obtained demonstrate that the enhanced sensitivity of PFC catecholamines to low-dose psychostimulants reflects an intrinsic property of the PFC.
MATERIALS AND METHODS
Animals and Surgery
Forty-six male Sprague-Dawley rats (260–280 g, Charles River, Wilmington, MA) had ad lib access to food and water on an 11 : 13-h light:dark cycle (lights on 0700 h). For microdialysis studies, probes were surgically implanted under isoflurane anesthesia. All procedures were in accordance with NIH guidelines and were approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Microdialysis and HPLC Analyses of NE and DA
In all cases, 1–2 days before testing, laboratory-made microdialysis probes were lowered into the PFC (A+3.2; L1.0; V-5.0 mm at an angle of 4° lateral), the core subregion of the NAc (A+1.7; L1.4; V-7.8 mm), or the MSA (A-0.7; L0.5; V-7.8 mm) as described previously (Berridge et al, 2006; Berridge and Stalnaker, 2002). No more than two regions were sampled per animal. Animals were housed in the testing chamber following surgery (see below) and artificial extracellular fluid (AECF; 147 mM NaCl, 1.3 mM CaCl2, 0.9 mM MgCl2, 2.5 mM KCl; pH 7.4) was perfused at a rate of 1.5 μl/min through PE20 tubing to a length of Spectra/Por hollow fiber (MW cutoff 13 000, o.d. 250 μm). The dialysis membrane was sealed and attached to the PE20 tubing with epoxy. The approximate length of functional dialysis membrane was 4 mm for PFC, 3 mm for MSA, and 2 mm for NAc. This active membrane began immediately above the ∼1 mm epoxy plug located at the most ventral tip of the probe. Fused silica (150 mm o.d., 75 mm i.d.) provided outflow of dialysate to a sample collection vial outside the testing chamber. The average recovery for probes was as follows: PFC, 10.3±0.3% for NE (n=20) and 11.2±0.5% for DA (n=21); MSA, 8.8±0.8% for NE (n=7) and 10.1±1.1% for DA (n=7); NAc, 8.6±0.5% for DA (n=21).
DA and NE were measured in dialysate samples using HPLC with electrochemical detection. For these analyses, 20 μl aliquots were immediately injected following collection onto an HPLC-EC system consisting of an ESA Model 582 pump and an ESA 5100 A Coulochem II detector with 2 electrodes in series: −0.25 mV, +220 mV (Cell Model 5014B; ESA, Boston, MA). For DA, samples were injected onto a Velosep C18 100 × 3.2 mm column with a mobile phase consisting of 200 mM sodium phosphate (pH 3.0–4.5), 0.1 mM EDTA, 0.3 mM sodium octyl-sulfate, and 5% v/v methanol with the pump set at 0.6 ml/min. For NE, samples were injected onto an ion exchange column (ESA, MD-16) and the mobile phase consisted of 150 mM ammonium acetate (pH ∼6.0), 0.14 mM EDTA, 15% v/v methanol, and 5% acetonitrile with the pump set at 0.2 ml/min.
In all cases, 30-min samples were collected before and following local perfusion of methylphenidate hydrochloride (MPH; Sigma, St Louis, MO) dissolved in AECF via reverse dialysis. For the PFC and MSA, samples were split and analyzed for both DA and NE. Baseline values were determined from samples characterized by low levels of waking (ie, sleeping) and displaying less than 10% variation from the average value. The quantitation limit for both NE and DA (three times background noise) was ∼0.3 pg (20 μl samples). The mean baseline concentration of NE per sample was 1.4±0.1 pg within the PFC (n=23) and 1.5±0.2 pg within the MSA (n=7). The mean baseline concentration of DA per sample was 1.0±0.1 pg (n=22) within the PFC, 1.0±0.1 pg within the MSA (n=7), and 7.0±0.5 pg (n=22) within the NAc.
Microdialysis Experimental Procedure
Microdialysis sample collection was conducted in a Plexiglas testing chamber (32 × 32 × 40 cm) containing bedding and housed in a ventilated, sound-attenuating chamber (Berridge and Foote, 1996). Animals had ad lib access to food and water. Baseline samples were collected during the light phase of the circadian cycle between the hours of 0800 and 1300, identical to prior studies examining the effects of systemic MPH on extracellular catecholamines in the PFC, NAc, and MSA (Berridge et al, 2006). On the day of testing, at least four 30-min baseline dialysis samples were collected before switching the AECF-containing syringe to a syringe containing AECF+MPH (0.25, 1.0, and 4.0 μM). Following this switch, there was a 30-min delay before resuming sample collection to allow for pressure/flow-rate restabilization. Sample collection continued for an additional 4 h.
Statistical Analyses
A mixed-design three-way ANOVA for concentration (between subjects; three levels), region/neurotransmitter (between subjects; five levels), and time (within subjects; six levels, representing 6-1 h epochs) was used to analyze neurochemical data (see Figure 2). For each time epoch within a dose, pairwise post hoc analyses were conducted using independent t-tests. Matched-pair t-tests were also used to determine whether within a given group post-treatment measures differed significantly from the baseline epoch that immediately preceded MPH perfusion. To further characterize the neurochemical effects of the 1.0 μM MPH concentration, additional analyses were conducted to assess the effects of this concentration on catecholamines over an extended 90-min period (see Figure 3). For these latter analyses, drug-induced changes in DA across regions were examined using a one-way ANOVA (three levels, PFC, NAc, and MSA) followed by a Tukey’s HSD post hoc test, while NE effects were examined using an independent t-test (PFC vs MSA).
Histological Analyses and Data Selection
The placement of microdialysis probes was verified in 40 μm thick coronal sections stained with Neutral Red dye. Data from a given experiment were included only when histological analyses verified accurate probe placement within a target region and NE or DA concentrations were stable (<±10%) throughout baseline.
RESULTS
Effects of Local Application of MPH on NE and DA Within the PFC, NAc, and MSA
To determine whether low-dose MPH differentially impacts extracellular NE and DA levels across regions, we initially examined the effects of varying concentrations of locally perfused MPH (0.25, 1.0, and 4.0 μM) on NE and DA in the PFC and DA in the NAc (Figure 1). This first series of measurements indicated that the 1.0 μM concentration elicited differential actions across regions. Therefore, we extended our measurements for this concentration of MPH to NE and DA within the MSA, an area that receives moderate DA and NE innervation, similar to the PFC (Figure 1). This region permitted: (1) determining the degree to which subcortical NE differs in sensitivity from PFC NE; and (2) extending our analyses to a second subcortical DA terminal field that differs in the degree of DA innervation and DA transporter (DAT) density from the NAc. The number of data points per MPH concentration, region, and transmitter were (1) 0.25 μM: PFC NE, n=8; PFC DA, n=7; NAc DA, n=7; (2) 1.0 μM: PFC NE, n=7; MSA NE, n=7; PFC DA, n=7; NAc DA, n=7; MSA DA, n=7; (3) 4.0 μM, PFC NE, n=8; PFC DA, n=8; NAc DA, n=8. An omnibus ANOVA for concentration, time, and region indicated that MPH significantly increased NE and DA in a concentration- and region-dependent manner (Figure 2; concentration: F2,70=24.55, P<0.001; region: F4,70=2.07, P=0.09; concentration × region: F4,70=1.70, P=0.16; time: F5,350=224.39, P<0.001; time × concentration: F10,350=35.32, P<0.001; time × region: F20,350=1.94, P<0.05; time × concentration × region: F20,350=3.11, P<0.001).
Photomicrographs of Nissl-stained tissue sections depicting placement of dialysis probes within prefrontal cortex (PFC; a), nucleus accumbens (NAc; b), and medial septal area (MSA; c). For PFC, probes spanned the majority of the dorsoventral extent of the medial PFC. For NAc, probes were generally placed within the central portion corresponding to the core subdivision. For MSA, probes were placed medially, largely within the medial septum and diagonal band of Broca. Arrows indicate probe track and point toward midline. ac, anterior commissure; CC, corpus callosum; LV, lateral ventricle.
Regional effects of local application of methylphenidate (MPH) on extracellular levels of norepinephrine (NE) and dopamine (DA). Mean (±SEM) NE and DA levels expressed as percentage of baseline following local application of MPH at varying concentrations via reverse dialysis within the prefrontal cortex (PFC), nucleus accumbens (NAc), and medial septal area (MSA) are shown. (a–c) The effects of 0.25, 1.0, and 4.0 μM MPH, respectively, on DA (left panels) in the PFC, NAc and MSA and NE (right panels) in the PFC and MSA (1.0 μM only) are depicted. Values represent the average of two 30-min samples collected 2 h before (negative numbers) and beginning 30-min after MPH application (positive numbers). The 1.0 μM concentration elicited significantly larger increases in DA and NE relative to the subcortical regions in the second and third 60-min collection epochs. *P<0.05, **P<0.01 compared with Sample -1; +P<0.05, ++P<0.01 compared with NAc DA within the same sample epoch; #P<0.05 compared with MSA NE within the same sample epoch.
Regional Effects of MPH
Given the highly significant time × concentration × region interaction, additional analyses examined the effects of a given concentration on NE/DA levels across regions for each post-treatment time period. These analyses indicated that local application of 1.0 μM MPH, but not 0.25 or 4.0 μM MPH, resulted in elevations in PFC catecholamines similar to that seen with systemic administration of clinically relevant doses (eg, increases of 100–200%; Berridge et al, 2006). Moreover, as shown in Figure 2, the 1.0 μM dose exerted preferential actions on PFC catecholamines relative to the other two regions, also similar to that seen with systemic administration of clinically relevant doses.
Specifically, at the 1.0-μM concentration, MPH produced significantly larger increases in PFC DA than either NAc DA or MSA DA in the second and third 60-min post drug collection epochs (NAc, Post-2, t12=3.39, P=0.005; Post-3, t12=2.58 P=0.027; MSA, Post-2, t12=2.25, P<0.05; Post-3 t12=2.16 P=0.05). Similarly, for NE, this concentration of MPH elicited significantly larger increases in PFC NE relative to the NE in the MSA in the second 60-min collection epoch (t12=2.71, P=0.019) with a trend for a significant difference in the third 60-min collection epoch (t12=1.69 P=0.12). These differences across regions observed at the 1.0 μM concentration are reduced in later time points as the subcortical catecholamine levels continue to increase while levels in the PFC reach a steady state. At the 1.0-μM concentration, the effects of MPH on NE and DA within the PFC were not significantly different.
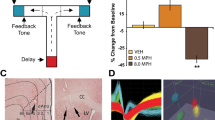
To further characterize the regionally selective effects of 1.0 μM MPH, we examined the effects of this concentration on NE and DA levels averaged over a 90-min period spanning 90–180 min after MPH application. As shown in Figure 3, MPH maximally increased extracellular PFC DA by ∼100% compared with an ∼50% increase in DA within both the NAc and MSA (F2,20=6.04, P<0.01). For NE, MPH increased PFC NE ∼120% above baseline compared with an increase of ∼50–60% within the MSA (Figure 3; t12=2.52, P<0.05).
Locally administered methylphenidate (MPH) increases dopamine (DA) and norepinephrine (NE) preferentially within the prefrontal cortex (PFC). The effects of local application of 1.0 μM MPH on extracellular levels of DA and NE within the PFC, nucleus accumbens (NAc), and medial septal area (MSA) are shown. Data are an average (±SEM) of three 30-min samples collected 90–180 min following MPH application and are expressed as percent above baseline. At this dose, MPH produced only modest increases in DA and NE levels (∼50–60%) in subcortical regions (NAc and MSA), while eliciting significantly larger increases in DA and NE levels (∼100–120%) within the PFC. A similar pattern of effects is seen with systemic administration of MPH (Berridge et al, 2006). *P<0.05 relative to NAc DA and +P<0.05 relative to MSA NE.
DISCUSSION
Clinically relevant and cognition-enhancing doses of psychostimulants produce larger increases in extracellular levels of DA and NE in the PFC than in cortical and subcortical regions outside the PFC, including regions associated with the arousing and motor-activating effects of these drugs (Berridge et al, 2006; Drouin et al, 2007; Kuczenski and Segal, 2001; Kuczenski and Segal, 2002). At higher and behaviorally activating doses, this preferential targeting of PFC catecholamines does not occur (Kuczenski et al, 1995; Moghaddam et al, 1993). This and other evidence indicates that the preferential elevation of PFC catecholamines by low-dose psychostimulants contributes to their ability to promote PFC-dependent cognition while lacking the locomotor-activating and arousal-promoting effects typically associated with these drugs (Berridge et al, 2006; Spencer et al, 2012). However, the circuitry responsible for the selective targeting of PFC catecholamines by low-dose psychostimulants has been unclear.
The current studies demonstrate that when applied directly to the PFC at a concentration that elevates catecholamines in a range seen with systemic administration of clinically relevant doses (100–200%), MPH produced significantly smaller increases in DA and NE in the two subcortical regions examined (∼50%). These observations indicate that the enhanced sensitivity of PFC catecholamines to clinically relevant doses of psychostimulants reflects, at least in part, mechanisms contained within the PFC. This information provides new insight into the neurobiology underlying the neurochemical and cognitive effects of cognition-enhancing doses of psychostimulants. Moreover, these studies provide critical information for future research aimed at understanding the cellular mechanisms responsible for the enhanced sensitivity of PFC catecholamines to clinically relevant doses of psychostimulants and other drugs used in the treatment of ADHD (eg, selective NE reuptake inhibitors (SNRIs); Bymaster et al, 2002).
The Pharmacology of ADHD: Preferential Targeting of the PFC
Neuropsychological and imaging evidence suggests a prominent involvement of the PFC in ADHD (for review, Arnsten and Castellanos, 2002). Consistent with this, drugs used to treat ADHD, including psychostimulants, SNRIs and α2-receptor agonists, improve an array of PFC-dependent processes (Chamberlain et al, 2007; Diamond, 2005; Mehta et al, 2001; Turner et al, 2005). Neurochemically, low and clinically relevant doses of MPH preferentially increase extracellular levels of NE and DA within the PFC relative to other cortical and subcortical regions (for review, Berridge and Devilbiss, 2011). Moreover, despite their selectivity for the NET, SNRIs used in the treatment of ADHD (desipramine and atomoxetine) also elevate both NE and DA in the PFC (Bymaster et al, 2002; Carboni et al, 2006; Carboni et al, 1990; Yamamoto and Novotney, 1998). These observations suggest that the cognition-enhancing/therapeutic effects of drugs used to treat ADHD involve increased catecholamine signaling within the PFC. Consistent with this hypothesis, direct infusion of MPH into the PFC, but not dorsomedial striatum, improves working memory performance (Spencer et al, 2012). This cognition-enhancing action of intra-PFC MPH is similar to that seen with intra-PFC infusion of clinically efficacious α2 agonists (eg, guanfacine; for review, Arnsten, 2009).
These observations indicate that the cognition-enhancing actions of low-dose psychostimulants involve drug-induced elevations in PFC catecholamines. Conversely, the fact that clinically relevant doses of psychostimulants exert only a modest impact on circuitry associated with psychostimulant-induced arousal (eg, MSA NE; Berridge, 2006) and motor activation (eg, NAc; Kelley et al, 1989) is consistent with their minimal arousal-promoting and motor-activating effects. Moreover, the fact that clinically relevant doses of psychostimulants exert a modest impact on DA levels in the NAc, a key region involved in the reinforcing effects of psychostimulants, is also consistent with evidence indicating that the clinical use of psychostimulants does not increase, and may reduce, the liability for drug abuse in ADHD populations (Biederman, 2003). Collectively, these observations indicate that the preferential targeting of PFC catecholamines contributes to the therapeutic actions of psychostimulants seen in the treatment of ADHD.
Potential Mechanisms Underlying the Preferential Targeting of PFC Catecholamines
The current studies identify the PFC as a site involved in the enhanced sensitivity of PFC catecholamines. Currently, the cellular mechanisms within this region responsible for the enhanced sensitivity of catecholamines to low-dose psychostimulants remain to be elucidated. One possible mechanism involves the tight relationship between DA and NE clearance within the PFC. Anatomical studies demonstrate a limited density of the DAT in the PFC (Sesack et al, 1998). Additional evidence indicates that the NET has a prominent role in DA clearance within the PFC. Indeed, it has long been known that the NET displays a higher affinity for DA than does the DAT (Giros et al, 1994; Gu et al, 1994). Consequently, in regions with minimal DAT (eg, PFC), drugs that block the NET increase both NE and DA while having no significant impact on DA levels in regions with high DAT density and low NET density (eg, NAc; Bymaster et al, 2002; Carboni et al, 1990). Conversely, recent studies demonstrate that a highly selective DAT inhibitor also elevates both DA and NE in the PFC (Schmeichel et al, 2012). These observations suggest that NE and DA binding to the NET in the PFC is competitive, with elevations in extracellular levels of one transmitter resulting in elevations of the other. Such a feed-forward mechanism could contribute to the differential sensitivity of PFC catecholamines to low-dose psychostimulants relative to other regions.
In addition to the tight linkage of NE and DA clearance in the PFC, amino acids and other transmitters are known to modulate catecholamine/monoamine release via presynaptic receptors (Bonanno et al, 1989; Galli et al, 1991; Raiteri et al, 1989). Thus, psychostimulant-induced alterations in amino-acid signaling or other neurotransmitters could exert region-specific modulation of extracellular catecholamine levels.
Although the pattern of changes in extracellular NE and DA across regions produced by locally perfused MPH was largely similar to that seen with systemic administration of clinically relevant doses, there was one exception. Namely, when administered systemically, clinically relevant doses produce significantly larger increases in extracellular levels of NE (∼200%) than DA (∼100%) in the PFC (relative to vehicle treatment). However, in the current studies, perfusion of 1.0 μM MPH directly into the PFC elicited relatively comparable increases in PFC NE and DA, with the magnitude of the increase in NE (∼120%) smaller than that seen with systemic administration of clinically relevant doses. This difference could reflect the fact that extracellular levels of NE are significantly more sensitive to arousal state than are DA levels (Berridge et al, 2006; Berridge and Stalnaker, 2002) and that systemic administration of clinically relevant doses of MPH exert modest wake-promoting actions (Berridge et al, 2006). Alternatively, the current studies may have missed the optimal concentration for maximally elevating PFC NE. Finally, drug action outside the PFC may modulate the effects of clinically relevant doses on NE and/or DA levels in the PFC.
Psychostimulant Action Outside the PFC May Contribute to Their Therapeutic Efficacy in ADHD
The available information indicates that the therapeutic and cognition-enhancing actions of low-dose psychostimulants involve alterations in PFC catecholamines. However, it is important to note that this does not preclude actions outside the PFC in the cognitive/therapeutic effects of these drugs. Anatomical, electrophysiological, and pharmacological studies demonstrate that the PFC represents one node in an extended frontostriatal network that guides goal-directed behavior (Voorn et al, 2004). Moreover, there is strong evidence that ADHD is associated with dysfunction in this broader frontostriatal circuitry (Castellanos and Tannock, 2002). Thus, psychostimulant-induced elevation in striatal DA signaling may contribute to the therapeutic and cognition-enhancing actions of psychostimulants. Consistent with this, psychostimulants are generally viewed as more effective than selective NE reuptake blockers (atomoxetine and desipramine) in treating ADHD. Although NE reuptake blockers elevate both NE and DA in the PFC, they have minimal effects on striatal DA (Bymaster et al, 2002). These observations suggest that modest increases in striatal DA signaling elicited by clinically relevant doses of psychostimulants may contribute to their clinical efficacy. In prior work, we demonstrated that microinfusion of MPH into the dorsomedial, but not into ventromedial PFC, improves PFC-dependent cognition as measured in a spatial working memory task (Spencer et al, 2012). In the rat, the dorsomedial PFC sends a prominent projection to the dorsomedial striatum (Gabbott et al, 2005), a region that is necessary for accurate performance of ‘PFC-dependent’ working memory tasks (Spencer et al, 2012). Nonetheless, MPH infusion into this region had no noticeable effect on performance in this task (Spencer et al, 2012).
The NAc is implicated in the regulation of impulsivity (Cardinal et al, 2001; Christakou et al, 2001) and working memory (Floresco et al, 1999), as well as the pathophysiology of ADHD (for review, Castellanos and Tannock, 2002). Functional imaging studies indicate that MPH-induced improvement in certain cognitive/behavioral tasks is associated with alterations in NAc activity (Dodds et al, 2008; Seidman et al, 2005). Moreover, MPH-induced changes in DA receptor occupancy in the ventral striatum are correlated with the magnitude of MPH-induced improvement in a spatial working memory task (Clatworthy et al, 2009). However, it should be noted that although it is currently not possible to image DA receptor/transporter occupancy in the PFC, evidence strongly indicates that there is likely a similar or stronger association between MPH-induced changes in DA/NE receptor occupancy within the PFC and MPH-induced changes in cognition (Berridge et al, 2006). Nonetheless, collectively, these observations indicate that the cognitive/therapeutic effects low-dose psychostimulants may involve actions within the NAc. Ongoing studies in our laboratory are testing this hypothesis.
References
Arnsten AF (2009). Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs 23 (Suppl 1): 33–41.
Arnsten AFT, Castellanos FX (2002). Neurobiology of attention regulation and its disorders. In: Martin A, Scahill L, Charney D, Leckman J, (eds) Textbook of Child and Adolescent Psychopharmacology. Oxford University Press: New York. pp 99–109.
Berridge CW (2006). Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology 31: 2332–2340.
Berridge CW, Devilbiss DM (2011). Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol Psychiatry 69: e101–e111.
Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B et al (2006). Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60: 1111–1120.
Berridge CW, Foote SL (1996). Enhancement of behavioral and electroencephalographic indices of waking following stimulation of noradrenergic beta-receptors within the medial septal region of the basal forebrain. J Neurosci 16: 6999–7009.
Berridge CW, Stalnaker TA (2002). Relationship between low-dose amphetamine-induced arousal and extracellular norepinephrine and dopamine levels within prefrontal cortex. Synapse 46: 140–149.
Biederman J (2003). Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry 64 (Suppl 11): 3–8.
Bonanno G, Fontana G, Fedele E, Robino G, Raiteri M (1989). Presynaptic mechanisms underlying the gamma-aminobutyric acid-evoked receptor-independent release of [3H]norepinephrine in rat hippocampus. J Neurochem 52: 1854–1858.
Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH et al (2002). Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27: 699–711.
Carboni E, Silvagni A, Vacca C, Di CG (2006). Cumulative effect of norepinephrine and dopamine carrier blockade on extracellular dopamine increase in the nucleus accumbens shell, bed nucleus of stria terminalis and prefrontal cortex. J Neurochem 96: 473–481.
Carboni E, Tanda GL, Frau R, Di CG (1990). Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem 55: 1067–1070.
Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ (2001). Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292: 2499–2501.
Castellanos FX, Tannock R (2002). Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nat Rev Neurosci 3: 617–628.
Chamberlain SR, Del CN, Dowson J, Muller U, Clark L, Robbins TW et al (2007). Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry 62: 977–984.
Christakou A, Robbins TW, Everitt BJ (2001). Functional disconnection of a prefrontal cortical-dorsal striatal system disrupts choice reaction time performance: implications for attentional function. Behav Neurosci 115: 812–825.
Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L et al (2009). Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci 29: 4690–4696.
Diamond A (2005). Attention-deficit disorder (attention-deficit/ hyperactivity disorder without hyperactivity): a neurobiologically and behaviorally distinct disorder from attention-deficit/hyperactivity disorder (with hyperactivity). Dev Psychopathol 17: 807–825.
Dodds CM, Muller U, Clark L, van LA, Cools R, Robbins TW (2008). Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci 28: 5976–5982.
Drouin C, Wang D, Waterhouse BD (2007). Neurophysiological actions of methylphenidate in the primary somatosensory cortex. Synapse 61: 985–990.
Floresco SB, Braaksma DN, Phillips AG (1999). Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci 19: 11061–11071.
Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ (2005). Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492: 145–177.
Galli T, Godeheu G, Artaud F, Desce JM, Pittaluga A, Barbeito L et al (1991). Specific role of N-acetyl-aspartyl-glutamate in the in vivo regulation of dopamine release from dendrites and nerve terminals of nigrostriatal dopaminergic neurons in the cat. Neuroscience 42: 19–28.
Gamo NJ, Wang M, Arnsten AF (2010). Methylphenidate and atomoxetine enhance prefrontal function through alpha2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry 49: 1011–1023.
Giros B, Wang YM, Suter S, McLeskey SB, Pifl C, Caron MG (1994). Delineation of discrete domains for substrate, cocaine, and tricyclic antidepressant interactions using chimeric dopamine-norepinephrine transporters. J Biol Chem 269: 15985–15988.
Greenhill LL (2001). Clinical effects of stimulant medication in ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX, (eds) Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. Oxford University Press: New York. pp 31–71.
Gu H, Wall SC, Rudnick G (1994). Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem 269: 7124–7130.
Kelley AE, Gauthier AM, Lang CG (1989). Amphetamine microinjections into distinct striatal subregions cause dissociable effects on motor and ingestive behavior. Behav Brain Res 35: 27–39.
Kuczenski R, Melega WP, Cho AK, Segal DS (1997). Extracellular dopamine and amphetamine after systemic amphetamine administration: comparison to the behavioral response. J Pharmacol Exp Ther 282: 591–596.
Kuczenski R, Segal DS (2001). Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther 296: 876–883.
Kuczenski R, Segal DS (2002). Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci 22: 7264–7271.
Kuczenski R, Segal DS, Cho AK, Melega W (1995). Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci 15: 1308–1317.
Mehta MA, Sahakian BJ, Robbins TW (2001). Comparative psychopharmacology of methylphenidate and related drugs in human volunteers, patients with ADHD, and experimental animals. In: Solanto MV, Arnsten AFT, Castellanos FX, (eds) Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. Oxford University Press: New York. pp 303–331.
Moghaddam B, Berridge CW, Goldman-Rakic PS, Bunney BS, Roth RH (1993). In vivo assessment of basal and drug-induced dopamine release in cortical and subcortical regions of the anesthetized primate. Synapse 13: 215–222.
Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002). Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22: 389–395.
Raiteri M, Marchi M, Maura G, Bonanno G (1989). Presynaptic regulation of acetylcholine release in the CNS. Cell Biol Int Rep 13: 1109–1118.
Rapoport JL, Inoff-Germain G (2002). Responses to methylphenidate in attention-deficit/hyperactivity disorder and normal children: update 2002. J Atten Disord 6 (Suppl 1): S57–S60.
Schmeichel B, Zemlan F, Berridge CW (2012). A selective dopamine reuptake inhibitor imporves prefrontal cortex-dependent cognitive function: potential relevance to attention deficit hyperactivity disorder. Neuropharmacology 64: 321–328.
Seidman LJ, Biederman J, Monuteaux MC, Valera E, Doyle AE, Faraone SV (2005). Impact of gender and age on executive functioning: do girls and boys with and without attention deficit hyperactivity disorder differ neuropsychologically in preteen and teenage years? Dev Neuropsychol 27: 79–105.
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI (1998). Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 18: 2697–2708.
Solanto MV (2001). Attention-Deficit/Hyperactivity Disorder. In: Solanto MV, Arnsten AFT, Castellanos FX, (eds) Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. Oxford University Press: New York. pp 3–30.
Spencer RC, Klein RM, Berridge CW (2012). Psychostimulants act within the prefrontal cortex to improve cognitive function. Biol Psychiatry 72: 221–227.
Spencer T, Biederman J, Wilens T (2004). Nonstimulant treatment of adult attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 27: 373–383.
Turner DC, Blackwell AD, Dowson JH, McLean A, Sahakian BJ (2005). Neurocognitive effects of methylphenidate in adult attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 178: 286–295.
Valentini V, Frau R, Di CG (2004). Noradrenaline transporter blockers raise extracellular dopamine in medial prefrontal but not parietal and occipital cortex: differences with mianserin and clozapine. J Neurochem 88: 917–927.
Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM (2004). Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27: 468–474.
Yamamoto BK, Novotney S (1998). Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem 71: 274–280.
Acknowledgements
This work was supported by PHS grant MH081843.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Berridge has received expert witness fees from Teva Pharmaceutical, Activis, Aurobindo Pharmaceuticals, Mylan Pharmaceuticals, and Apotex in the past 3 years. Dr Schmeichel has no conflict of interest.
Rights and permissions
About this article
Cite this article
Schmeichel, B., Berridge, C. Neurocircuitry Underlying the Preferential Sensitivity of Prefrontal Catecholamines to Low-Dose Psychostimulants. Neuropsychopharmacol 38, 1078–1084 (2013). https://doi.org/10.1038/npp.2013.6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.6
Keywords
This article is cited by
-
An exploratory analysis of the performance of methylphenidate regimens based on a PKPD model of dopamine and norepinephrine transporter occupancy
Journal of Pharmacokinetics and Pharmacodynamics (2023)
-
Movement disorders and use of risperidone and methylphenidate: a review of case reports and an analysis of the WHO database in pharmacovigilance
European Child & Adolescent Psychiatry (2021)
-
Evidence That Methylphenidate Treatment Evokes Anxiety-Like Behavior Through Glucose Hypometabolism and Disruption of the Orbitofrontal Cortex Metabolic Networks
Neurotoxicity Research (2021)
-
Repeated restraint stress potentiates methylphenidate and modafinil-induced behavioral sensitization in rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2020)
-
Direct comparison of the acute subjective, emotional, autonomic, and endocrine effects of MDMA, methylphenidate, and modafinil in healthy subjects
Psychopharmacology (2018)