Abstract
Diffusion MRI investigations in schizophrenia provide evidence of abnormal white matter (WM) microstructural organization as indicated by reduced fractional anisotropy (FA) primarily in interhemispheric, left frontal and temporal WM. Using tract-based spatial statistics (TBSS), we examined diffusion parameters in a sample of patients with severe chronic schizophrenia. Diffusion MRI data were acquired on 19 patients with chronic severe schizophrenia and 19 age- and gender-matched healthy controls using a 64 gradient direction sequence, (b=1300 s/mm2) collected on a Siemens 1.5T MRI scanner. Diagnosis of schizophrenia was determined by Diagnostic and Statistical Manual for Mental Disorders 4th Edition (DSM-IV) Structured Clinical Interview for DSM disorder (SCID). Patients were treatment resistance, having failed to respond to at least two antipsychotic medications, and had prolonged periods of moderate to severe positive or negative symptoms. Analysis of diffusion parameters was carried out using TBSS. Individuals with chronic severe schizophrenia had significantly reduced FA with corresponding increased radial diffusivity in the genu, body, and splenium of the corpus callosum, the right posterior limb of the internal capsule, right external capsule, and the right temporal inferior longitudinal fasciculus. There were no voxels of significantly increased FA in patients compared with controls. A decrease in splenium FA was shown to be related to a longer illness duration. We detected widespread abnormal diffusivity properties in the callosal and temporal lobe WM regions in individuals with severe chronic schizophrenia who have not previously been exposed to clozapine. These deficits can be driven by a number of factors that are indistinguishable using in vivo diffusion-weighted imaging, but may be related to reduced axonal number or packing density, abnormal glial cell arrangement or function, and reduced myelin.
Similar content being viewed by others
INTRODUCTION
Schizophrenia is a chronic and disabling mental disorder (Jablensky, 2000) associated with gray matter (GM) and white matter (WM) abnormalities. Recent meta-analyses of voxel-based imaging studies showed widespread clusters of GM volume reductions with no regions of volume increases, and widespread WM deficity in schizophrenia (Bora et al, 2011). Abnormalities in WM detected using diffusion MRI may indicate alterations in commissural and association fibers connecting GM regions (Ellison-Wright and Bullmore, 2009), Moreover, progressively more severe deficits in WM anisotropy have been found in cross-sectional studies comparing first episode and chronic patients (Friedman et al, 2008; Kong et al, 2011).
Standard structural MRI lacks orientational contrast making it impossible to distinguish between different WM tracts. Diffusion-weighted MRI or DWI, however, allows us to indirectly probe the microstructural organization of WM (Le Bihan et al, 2001), while providing orientational information to visualize and differentiate between large fiber bundles. DWI techniques measure the diffusion rate of water molecules moving within and between axonal bundles. Diffusion is larger in the directions parallel to the axon bundle, than in the plane perpendicular to these axons. Measuring diffusion in multiple directions with diffusion tensor imaging (DTI) therefore allows us to infer differences in the microstructural organization or orientation of WM bundles between groups of subjects (Basser et al, 1994). Fractional anisotropy (FA), an outcome parameter of DTI, is determined by a combination of factors including fiber orientation, axonal packing density, membrane permeability, and to a lesser extent the degree of myelination (Beaulieu, 2002; Le Bihan et al, 2001; Pierpaoli et al, 1996; Tournier et al, 2011). DTI outcome parameters also include axial diffusivity (AD) (diffusion in the principal direction of an axon bundle) and radial diffusivity (RD) (diffusion perpendicular to the direction of an axon bundle). AD has been shown to be modulated by axonal degeneration, whereas RD is more specifically related to changes in the myelin sheath (Song et al, 2002).
In schizophrenia, diffusion MRI studies have shown significant decrease in FA in multiple WM fiber bundles relative to healthy controls using whole brain (Mori et al, 2007b; Rotarska-Jagiela et al, 2009; Schlosser et al, 2007; Shergill et al, 2007; Sussmann et al, 2009), restricted voxel-based (Douaud et al, 2007; Karlsgodt et al, 2008; Lim et al, 1999) and reconstructed tract-based (McIntosh et al, 2008; Phillips et al, 2009; Whitford et al, 2010) analysis methods. Longitudinal studies highlight the progressive nature of these deficits. In WM, a single study of individuals experiencing their first psychotic episode did not detect microstructural changes (as assessed by DTI) in the corpus callosum relative to healthy controls (Price et al, 2005). Studies including both first episode and chronic patients showed that patients with chronic disease had significantly reduced FA compared with controls, reaching only a trend level in first episode patients (Friedman et al, 2008; Kong et al, 2011). This implies a progressive nature associated with illness duration in schizophrenia, as previously reported in a structural study investigating GM and WM volumes reporting a progressive reduction in the frontal WM volume in patients (Ho et al, 2003).
The most consistently implicated WM bundles in chronic schizophrenia samples include the genu of the corpus callosum (Kong et al, 2011; Kubicki et al, 2008; Rotarska-Jagiela et al, 2008; Whitford et al, 2010), the splenium of the corpus callosum (Agartz et al, 2001; Foong et al, 2000), anterior cingulate (Camchong et al, 2011; Sun et al, 2003; Wang et al, 2004), and anterior limb of the internal capsule (ALIC) (Buchsbaum et al, 1998; Sussmann et al, 2009). However, none of these studies were specifically carried out on a treatment-resistant clozapine-naive group, and clozapine has previously been reported to cause structural changes in the brain (Ho et al, 2003; Molina et al, 2005; Scheepers et al, 2001).
However, other studies contradict FA reductions in schizophrenia and report no significant FA difference in the genu but only in the splenium (Agartz et al, 2001; Foong et al, 2000), and no FA difference in any WM region (Foong et al, 2002; Kito et al, 2009; Levitt et al, 2010; Wang et al, 2003). Discrepancies in these findings could be attributed to methodological differences, subjective rater-based variation between studies such as region of interest (ROI) placement, and clinical heterogeneity including stage of illness and medication exposure. The contribution of long-term exposure to antipsychotic medication, further psychotic episodes, and illness contribute to these differences is unclear and difficult to examine explicitly.
In the present study, we examine changes in WM FA, radial and AD in a relatively clinically homogenous group of treatment-resistant individuals with severe chronic schizophrenia.
MATERIALS AND METHODS
Participants
DTI data were acquired from 19 individuals with severe chronic schizophrenia and 19 gender- and aged-matched healthy controls (Table 1). Patients were recruited from the in- and out-patient units in the University College Hospital Galway (UCHG) and in the catchment area of HSE West of Ireland.
All patients were diagnosed by experienced psychiatrists, using the Structured Clinical Interview for DSM disorders (SCID) (First et al, 2002b), as meeting the criteria for schizophrenia per the Diagnostic and Statistical Manual for Mental Disorders 4th Edition text revision (DSM-IV-TR) (Association AP, 2000). In order to provide information about symptom severity and functioning, the Positive and Negative Symptom Scale (PANSS) (Kay et al, 1987) and Global Assessment of Functioning Score (GAF) (Hall, 1995) were administered for each patient. All patients with severe chronic schizophrenia were treatment resistant at the time of scanning and were being considered for treatment with clozapine, an atypical antipsychotic medication licensed for treatment resistance. Treatment resistance was defined as the failure to respond to at least two antipsychotic medications, one of which an atypical, with a prolonged period of moderate to severe positive and/or negative symptoms (mean duration of illness=14.42 years+8.16, range=4–39 years). At the time of scanning, all patients were medicated with atypical antipsychotics (olanzapine n=10, aripiprazole n=4, paliperidone n=2, quetiapine n=2, and amisulpiride n=1), with some on two or more medications (atypical n=10, atypical+SSRI n=4, atypical+TCA n=1, and atypical+IM typical every 2 weeks n=4). No patients had commenced clozapine at the time of scanning.
The control group consisted of 19 participants with no current or past axis I or II disorders (DSM-IV-TR) and were screened using the SCID-Non-Patient Version (First et al, 2002a) and matched to the patients for age and gender. Controls were excluded if there was a personal or family history of a psychiatric illness.
In addition, all participants were subject to the following exclusion criteria: neurological disorders, learning disability, comorbid substance or alcohol misuse, a history of a head injury resulting in loss of consciousness >5 min, and general MRI contraindications including pregnancy. Ethical approval was granted by both the University College Hospital Galway and the National University of Ireland Galway Ethics committees, and written informed consent was obtained from each participant.
Image Acquisition
DTI data were acquired on a 1.5T Siemens Magnetom Symphony MRI scanner (Erlangen, Germany), utilizing an Echo Planar Imaging (EPI) based diffusion sequence acquired using parallel imaging (GRAPPA, factor 2), 64 independent diffusion gradient directions, b=1300 s/mm2, with 7 reference non-diffusion-weighted images (b-value=0 s/mm2), TR=8100 ms, TE=95 ms, in-plane voxel size of 2.5 × 2.5 mm, and a slice thickness of 2.5 mm, and SNR of b=1000 s/mm2 images >20. After scout images, the total imaging time was 10.24 min for the diffusion MRI sequence. Diffusion parameters were based on the study by Jones et al (1999), outlining the optimal strategies for measuring diffusion in anisotropic systems by MRI .
Image Processing and Statistics
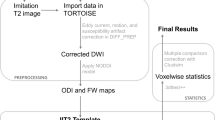
ExploreDTI (Leemans A JB and Jones, 2009) was used to process the diffusion MRI data and consisted of the following steps: (i) correction for motion and eddy current induced geometric distortions with rotation of the b-matrix to ensure the orientation information is correctly preserved (Leemans and Jones, 2009); (ii) estimation of the diffusion tensor using a robust nonlinear regression method (Chang et al, 2005); and (iii) calculation of FA, AD, and RD maps. Subsequently, voxel-based statistical analysis was carried out using the tract-based spatial statistics (TBSS) approach (Smith et al, 2006) as follows: FA images were nonlinearly registered using FNIRT (Anderson et al, 2007) to the MNI152 standard space, resulting in a standard space version of each individual FA image. Next, a study-specific thinned mean FA skeleton (threshold of 0.2 to exclude voxels of GM, CSF, and WM voxels with a low FA, which is associated with less certainty) was generated to represent the centre of all WM FA voxels common to the group. Alignment of each individuals FA image to the skeleton is carried out by searching a given subject’s FA image in the (already-computed) perpendicular tract direction to find the maximum FA value and assign this value to the skeleton voxel. Voxel-wise statistics is then carried out using Randomise, a permutation-based nonparametric analysis program (Nichols and Holmes, 2002). Qualitative analysis was carried out at each step of TBSS in order to check image quality of original data, determining the correct FA threshold, and alignment of each individuals FA image to the mean FA skeleton. All analyses included age as a covariate to increase the sensitivity of the analysis and to detect real differences between the groups, as age has been shown to effect FA (Bendlin et al, 2010). Multiple comparison correction was applied using threshold-free cluster enhancement (TFCE) (Smith and Nichols, 2009) with a reporting cutoff of p<0.05 applied. TFCE enhances cluster-like structures in an image and negates the need for defining an initial cluster-forming threshold; no spatial smoothing is required.
The original FA nonlinear registration was applied to AD and RD maps, which were then projected onto the mean FA skeleton. Randomise voxel-based statistical analysis was carried out as above, incorporating TFCE correction for multiple comparisons, to test for a main effect on AD and RD.
In order to further investigate what was driving intergroup differences, mean FA was extracted from the clusters where the highest statistical group difference was identified using a p-value cutoff threshold of p<0.05; the anatomical regions to which these belonged were determined using the JHU white-matter tractography atlas (Mori and van Zijl, 2007a). These were compared with illness severity (PANSS) and illness duration using a partial correlation controlling for age. Statistical analyses were carried out using PASW Statistics 18 (© 2010, SPSS Inc.).
RESULTS
The demographic characteristics of the participants are given in Table 1. Voxels showing significantly reduced FA were identified using the tractography atlas (Mori and van Zijl, 2007a). FA was lower in the schizophrenic group relative to healthy controls (Figure 1) in the genu, body, and splenium of the corpus callosum, bilaterally in the temporal portions of the ILF, superior longitudinal fasciculus (SLF), external capsule, temporal uncinate fasciculus (UF), posterior limb of the internal capsule, the left ALIC, fornix, cerebellar peduncles, and the corticospinal tract at the level of the brainstem. There were no voxels of increased FA in patients compared with controls.
Regions of reduced fractional anisotropy (FA) in the schizophrenia relative to the control group. Clusters of voxels (p<0.05, threshold-free cluster enhancement, TFCE) with significantly reduced FA in chronic schizophrenia compared with controls in (a) the genu, body, and splenium of the corpus callosum, (b) the body of the corpus callosum and the superior longitudinal fasciculus (SLF) (more prominent on the right), and (c) in the ILF. Significant clusters (p<0.05, TFCE) are denoted in a red (p=0.05) to yellow (lowest p-value) color intensity scale given at the bottom of the figure and in radiological format.
RD was significantly higher in the patient group compared with controls (Figure 2). Voxels of significantly higher RD were located in the genu, body, and splenium of the corpus callosum, the right ILF, posterior limb of the internal capsule, and the external capsule. There were no voxels of increased RD in the control group vs chronic schizophrenia group. AD was not significantly different between the diagnostic groups.
Regions where radial diffusivity (RD) was higher in the schizophrenia than the control group in the (a) genu and splenium of corpus callosum, (b) body of the corpus callosum and R-SLF, and (c) in the R-ILF. Increased RD in the chronic schizophrenia compared with the control group were evident in the genu (726 mm3, T=0.31–3.64, median=1.78), body (1421 mm3, T=0.38–3.98, median=1.87), and splenium (723 mm3, T=0.23–4.22, median=2.06) of the corpus callosum (p<0.05, threshold-free cluster enhancement, TFCE). Significant clusters (p<0.05, TFCE) are denoted in a blue (p=0.05) to light blue (lowest p-value) color intensity scale given at the bottom of the figure and in radiological format.
Mean FA was extracted from the clusters where the highest statistical group difference was identified using a p-value cutoff threshold of p<0.05. This included the genu (1309 mm3, T=0.79–3.9, median=1.78), body (2069 mm3, T=0.79–5.2, median=1.87), and splenium (723 mm3, T=0.70–3.8, median=1.50) of the corpus callosum (Figure 3a). Illness duration was negatively related to FA in the splenium (cc=−0.561, p=0.015) (Figure 3b) but not in the genu (cc=−0.412, p=0.089) or body (cc=−0.288, p=0.247) of the corpus callosum in the schizophrenia group; however, this did not survive the Bonferroni correction for multiple comparisons. FA in these callosal regions did not relate significantly to illness severity (PANSS score n=19, range=17–89, mean=52.31, SD=17.46, genu cc=0.206 p=0.41, body cc=0.304 p=0.220, and splenium cc=−0.022 p=0.932). There was no correlation between patient FA and chlorpromazine equivalents for each patient’s medication status at the time of scanning.
(a) Median fractional anisotropy (FA) is reduced in three regions of the corpus callosum in the schizophrenia (squares) compared with control subjects (circle). (b) Correlation of the splenium FA and illness duration in chronic schizophrenia. (a) Reduced FA in the genu (1309 mm3, T=0.79–3.9, median=1.78), body (2069 mm3, T=0.79–5.2, median=1.87), and splenium (723 mm3, T=0.70–3.8, median=1.50) of the corpus callosum in the schizophrenia (squares) compared with control subjects (circle). (b) Correlation of the splenium FA and illness duration in chronic schizophrenia.
DISCUSSION
We detected interhemispheric, cortico-cortical association, brainstem, and brainstem level projection fiber decreases in FA (Figure 1), which may reflect deficits in the microstructural organization of WM in a group of clozapine-naive individuals with severe chronic schizophrenia. The FA reductions extended throughout the corpus callosum, and were present bilaterally in parietal portions of the SLF, temporal ILF and UF, the posterior limb of the internal capsule, and the external capsule. They were also evident in the left ALIC, the fornix, the posterior projections of the middle cerebellar peduncle, midline portions of the superior and inferior cerebellar peduncles, and in the brainstem level of the corticospinal tracts. For a subgroup of these regions, this deficit was accompanied by increased RD: the corpus callosum, temporal portions of the right ILF including a region overlapping the right UF, the right external capsule, and the right posterior limb of the internal capsule (Figure 2). There were no group differences in AD and no regions of greater FA or reduced RD in the schizophrenia group relative to controls. The changes detected in FA and RD in schizophrenia may reflect deficits in microstructural organization such as alterations to the cellular membrane, fiber density, and the myelin sheath, and may be negatively related to the duration of illness, as reported here in the splenium of the corpus callosum.
The degree of anisotropic diffusion in WM is most directly influenced by the relative arrangement and orientation of the fibers. Thus, reduced FA (or more precisely in this case, the increase in RD detected) implicates several possible factors including reduced axonal number or packing density and cellular membrane abnormalities that lead to increased permeability such as loss of myelin or inconsistent fiber orientation (Beaulieu, 2002). Other factors that may affect the estimation of the diffusion tensor metrics include partial volume averaging and the existence of ‘crossing fibers’ configurations (Alexander et al, 2001; Jeurissen et al, 2012; Vos et al, 2012; Vos et al, 2011; Wheeler-Kingshott and Cercignani, 2009).
The observed differences in diffusivity measures were most prominent across the corpus callosum and highest in the body (4.6% median T=1.9, range 0.79–5.2, Cohen’s d=0.98), the genu (3.5% median T=1.8, range 0.79–3.9, Cohen’s d=0.86), and the splenium (2.9% median T=1.52, Cohen’s d=1.41). The latter is consistent with the pathophysiology of the corpus callosum in schizophrenia as evidenced by previous in vivo diffusion (Bora et al, 2011; Foong et al, 2000; Kong et al, 2011; Kubicki et al, 2008; Patel et al, 2011). Postmortem studies have shown a gender-diagnosis effect of reduced axonal number (Highley et al, 1999a), oligodendrocyte density loss (Hof et al, 2003), and lower levels of immunoreactivity of oligodendrocyte-associated proteins in the genu of the corpus callosum (Flynn et al, 2003). Diffusion-weighted imaging has been relatively consistent in reporting reduced callosal FA in chronic schizophrenia (Douaud et al, 2007; Koch et al, 2010; Kubicki et al, 2008; Miyata et al, 2010; Mori et al, 2007b; Rotarska-Jagiela et al, 2008), including during remission (Koch et al, 2010) and less consistently in studies examining individuals at the first episode (Cheung et al, 2008; Gasparotti et al, 2009; Perez-Iglesias et al, 2010b; Peters et al, 2008; Price et al, 2005; Szeszko et al, 2005) or in earlier stages of illness (Davenport et al, 2010; Douaud et al, 2007; Kyriakopoulos and Frangou, 2009). Directly comparing first episode and chronic groups supports more severe changes in FA in the genu of the corpus callosum (Friedman et al, 2008; Kong et al, 2011), the left ILF (Friedman et al, 2008), and ALIC (Bora et al, 2011) in the chronic group including relative to the first episode group. By contrast, the large scale meta-analysis by Bora et al (2011) has reported the opposite, specifically for the genu of the corpus callosum, with FA being lower in the first episode relative to the chronic group with schizophrenia.
Four meta-analyses have been conducted on diffusion imaging findings in cross-sectional studies (Bora et al, 2011; Di et al, 2009; Ellison-Wright and Bullmore, 2009; Patel et al, 2011). The two earlier studies (Di et al, 2009; Ellison-Wright and Bullmore, 2009) performed meta-analyses of studies that used a voxel-based studies and detected deficits in the genu and splenium in one case (Ellison-Wright and Bullmore, 2009) but not in the other (Di et al, 2009). A recent study conducted two meta-analyses (Patel et al, 2011) to examine the genu and splenium of the corpus callosum in 213 controls and 202 patients and reported reduced FA in both regions that were significant in the splenium (effect size 0.53) but subthreshold for reduced FA for the genu (effect size 0.22). However, this study included only those studies (n=7) using a ROI approach. The fourth meta-analysis included 699 individuals with schizophrenia and 681 controls across 24 studies that used a voxel-based analysis approach and detected significant deficits in FA. Widespread deficits were detected, including interhemispheric fibers, specifically in the bilateral genu of the corpus callosum as well as medial frontal WM, the right ALIC, the right external capsule, and temporal lobe WM (Bora et al, 2011). Thus, it is plausible that the small effect size reported by Patel et al (2011) is due to the restriction of the meta-analysis to just seven studies. Not accounted for in these analyses are the few negative studies that exist. Two previous studies reported no differences in FA throughout the brain (Foong et al, 2002; Murakami et al, 2011); others have reported widespread lower FA but did not detect significant differences in the genu of the corpus callosum specifically (Agartz et al, 2001; Foong et al, 2000; Karlsgodt et al, 2008; Kubicki et al, 2008; Seal et al, 2008).
Several additional regions beyond the callosal body have been implicated in chronic schizophrenia including SLF (Rotarska-Jagiela et al, 2009; Rowland et al, 2009; Seok et al, 2007; Shergill et al, 2007; Szeszko et al, 2008), ILF (Ashtari et al, 2007; Lee et al, 2009; Phillips et al, 2009; Rotarska-Jagiela et al, 2009; Seal et al, 2008), UF (Burns et al, 2003; McIntosh et al, 2008; Miyata et al, 2010; Mori et al, 2007b; Seal et al, 2008), internal capsule (Buchsbaum et al, 1998; Lim et al, 1999), external capsule (Rotarska-Jagiela et al, 2009; Seal et al, 2008), cingulum (Kubicki et al, 2003; Mori et al, 2007b; Seok et al, 2007; Sun et al, 2003; Wang et al, 2004), fornix (Fitzsimmons et al, 2009; Kuroki et al, 2006), anterior commissure (Choi et al, 2011), arcuate fasciculus (Burns et al, 2003; Phillips et al, 2009), and cerebellar peduncles (Okugawa et al, 2006; Seok et al, 2007). Differences in RD are less examined, yet to date increases have been detected in the external capsule (Seal et al, 2008), in the left inferior temporal and left occipital lobe (Ashtari et al, 2007), in temporal WM (Koch et al, 2011), and in the middle cerebellar peduncle (Okugawa et al, 2006), and increased mean diffusivity has been detected in the splenium of the corpus callosum (Foong et al, 2000). The regions reported in these studies were also found in this work. For the most part, it appears that those studies with the largest sample sizes support the case of reduced microstructural organization in the interhemispheric, frontal deep and temporal WM fibers in schizophrenia, which is consistent with both the direction and regions implicated by the present findings.
The callosal microstructural deficits detected in vivo are supported by structural MRI and postmortem studies. The latter more specifically implicate several possible contributing factors to the abnormalities observed in schizophrenia in vivo studies. A structural MRI meta-analysis of 313 patients and 281 controls across 11 studies examining callosal morphometry detected reduced area but not length relative to controls (Woodruff et al, 1995). Postmortem studies have reported reductions in the corpus callosum (Highley et al, 1999a) and anterior commissure (Highley et al, 1999b) fiber number in schizophrenia and to a greater extent in females, although these findings are not consistently reported in the literature (Casanova et al, 1989), which may be a result of smaller sample sizes and increased heterogeneity in postmortem studies. Group differences detected in vivo in the UF (Burns et al, 2003; McIntosh et al, 2008; Miyata et al, 2010; Mori et al, 2007b; Seal et al, 2008) have not been supported by postmortem investigations, which report no differences fiber density and total fiber number in the UF between schizophrenia and control brains (Highley et al, 2002). In vivo and postmortem studies do concur on the right >left asymmetry in the UF. Finally, the fiber content (density multiplied by area) of the fornix did not differ significantly between schizophrenia and control groups (Chance et al, 1999).
The contributions of glial cell abnormalities to FA measured in vivo are not clear. Glial cells have a role in the activation of distinct intracellular pathways within neurons, to promote neuronal survival and axonal length (Wilkins et al, 2003). Abnormalities to glial cells could therefore plausibly contribute to dysmorphic axonal arrangement or packing density, which may affect the local anisotropy and diffusion signal. In schizophrenia, reduced oligodendrocyte-associated proteins (Flynn et al, 2003), fewer oligodendrocytes (Hof et al, 2003), and abnormal gene expression of key oligodendrocyte-related genes have been reported (Cotter et al, 2001; Flynn et al, 2003; Haroutunian et al, 2007; Hof et al, 2002; Tkachev et al, 2003). To date, the exact contribution of changes in axonal and support cell arrangement and packing on local anisotropy has not been directly assessed.
Finally, loss of myelin is a further potentially contributing factor in alterations in diffusion outcome parameters, presumably via increased cellular membrane permeability (Dyer, 2002). Diffusion anisotropy can be detected in the absence of myelin (Beaulieu, 2002), and the contribution of changes in myelin to altered diffusion measurements in vivo has been determined to be relatively low (Beaulieu, 2002, Beaulieu and Allen, 1994), with one study reporting a decrease of 20% in anisotropy in myelin-deficient animal models (Gulani et al, 2001). Nevertheless, in schizophrenia, myelination abnormalities have been demonstrated using MRI and T2 relaxation; reduced myelin water fraction was detected to a greater extent in the chronic than first episode groups and was localized to the genu of the corpus callosum and frontal WM (range 19–36%)(Flynn et al, 2003). Moreover, postmortem studies have detected 27% reduced immunoreactivity of myelin-associated glycoprotein (MAG) in the anterior frontal cortex (Flynn et al, 2003). Postmortem studies also provide evidence for myelin-related genetic abnormalities in schizophrenia indicating that oligodendrocyte and myelin genes are among the most prominently downregulated genes in schizophrenia (Hakak et al, 2001; Katsel et al, 2005). However, caution must be taken when interpreting in vivo dMRI results with reference to postmortem studies. Postmortem studies report results from more localized brain regions compared with the global dMRI analysis, as carried out in this study, and are usually carried out on smaller, more heterogeneous sample group compared with in vivo studies.
The analysis methods used in the above reviewed diffusion MRI studies in schizophrenia ranging from restricted to whole brain voxel-wise approaches, plus region of interest, based methods and the reconstruction of fibers by tracking methods. In addition, there is some variation in the acquisition parameters (number of directions, b-value), and in the eddy current and motion correction applied. Few studies to date have corrected for rotation of the b-matrix, which can introduce bias in orientation estimates and lead to reduced reliability of results (Leemans and Jones, 2009). We used a relatively high b-value (1300 s/mm2), and used 64 diffusion gradients for accurate diffusion tensor estimation (Jones, 2004). Analysis involved a restricted set of skeleton-deep WM voxels (TBSS), which avoids problems associated with over-smoothing that otherwise impacts on the final results and increases the effect of partial volume (Jones et al, 2005).
Objective comparison of findings across existing studies is complicated by the clinical heterogeneity of the samples included within chronic schizophrenia, medication history, and medication exposure at the time of scanning. We have included a relatively homogenous group with a clinical profile of severe enduring schizophrenia despite being medicated with atypical antipsychotics, and who have never previously been exposed to clozapine. It remains possible that the deficits observed in the present and previous studies may be due to a history or current exposure to psychotropic medications. However, the present findings confirm that these deficits are detectable before exposure to clozapine, which has been previously demonstrated to have structural effects on the brain (Ho et al, 2003; Molina et al, 2005; Scheepers et al, 2001). Nevertheless, it remains possible that typical and atypical antipsychotic medications contribute to deficits present at the time of scanning. Studies on drug-naive first episode patients also demonstrate reduced FA in patients with schizophrenia indicating that at least some of these WM abnormalities are likely to be related to the pathophysiology of schizophrenia (Cheung et al, 2008).
In conclusion, we detected localized reductions in FA with increases in RD in the corpus callosum and in the temporal lobe areas of cortico-cortical WM association tracts in a group of clozapine-naive treatment-resistant individuals with severe chronic schizophrenia that may be related negatively to the duration of illness. This may reflect reduced axonal number or packing density, cellular membrane abnormalities that increase permeability including possible loss of myelin, or inconsistent fiber orientation. These findings may be confounded by exposure to non-clozapine psychotropic medications; however, concurrent findings in studies involving subjects who are naive to psychotropic medication (Cheung et al, 2008; Gasparotti et al, 2009; Karlsgodt et al, 2009; Perez-Iglesias et al, 2010a; Zou et al, 2008) supporting the hypothesis that a portion of these deficits are due to the pathophysiology of schizophrenia. Postmortem studies provide support for a contribution of reduced axonal packing density, abnormal glial cell arrangement or function, and reduced myelin in schizophrenia. The extent to which these factors modulate the in vivo diffusion signal could be further informed by direct measurement of anisotropy in the healthy postmortem human brain and those with an antemortem diagnosis of schizophrenia.
FUNDING AND DISCLOSURE
This study was partly funded by The Wellcome Trust, NUI Galway College of Medicine Graduate Fellowship and Millennium Project Fund. GJB received honoraria for teaching from General Electric during the course of this work, and also acts as a consultant for IXICO. The authors declare no conflict of interest.
References
Agartz I, Andersson JL, Skare S. (2001). Abnormal brain white matter in schizophrenia: a diffusion tensor imaging study. Neuroreport 12: 2251–2254.
Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL (2001). Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med 45: 770–780.
Anderson JLR, Jenkinson M, Smith S (2007). Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2 www.fmrib.ox.ac.uk/analysis/techrep FMRIB Centre, Oxford, United Kingdom.
Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J et al (2007). Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry 64: 1270–1280.
Association AP (2000). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). 384 pp.
Basser PJ, Mattiello J, LeBihan D (1994). MR diffusion tensor spectroscopy and imaging. Biophysica J 66: 259–267.
Beaulieu C, Allen PS (1994). Determinants of anisotropic water diffusion in nerves. Magn Reson Med 31: 394–400.
Beaulieu C (2002). The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 15: 435–455.
Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Kastman EK, Thiel BW et al (2010). White matter in aging and cognition: a cross-sectional study of microstructure in adults aged eighteen to eighty-three. Dev Neuropsychol 35: 257–277.
Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ et al (2011). Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res 127: 46–57.
Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA et al (1998). MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport 9: 425–430.
Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC et al (2003). Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry 182: 439–443.
Camchong J, MacDonald AW 3rd, Bell C, Mueller BA, Lim KO (2011). Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull 37: 640–650.
Casanova MF, Zito M, Bigelow LB, Berthot B, Sanders RD, Kleinman JE (1989). Axonal counts of the corpus callosum of schizophrenic patients. J Neuropsychiatry Clin Neurosci 1: 391–393.
Chance SA, Highley JR, Esiri MM, Crow TJ (1999). Fiber content of the fornix in schizophrenia: lack of evidence for a primary limbic encephalopathy. Am J Psychiatry 156: 1720–1724.
Chang LC, Jones DK, Pierpaoli C (2005). RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med 53: 1088–1095.
Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L et al (2008). A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med 38: 877–885.
Choi H, Kubicki M, Whitford TJ, Alvarado JL, Terry DP, Niznikiewicz M et al (2011). Diffusion tensor imaging of anterior commissural fibers in patients with schizophrenia. Schizophr Res 130: 78–85.
Cotter DR, Pariante CM, Everall IP (2001). Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull 55: 585–595.
Davenport ND, Karatekin C, White T, Lim KO (2010). Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Res 181: 193–198.
Di X, Chan RC, Gong QY (2009). White matter reduction in patients with schizophrenia as revealed by voxel-based morphometry: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 33: 1390–1394.
Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J et al (2007). Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130: 2375–2386.
Dyer CA (2002). The structure and function of myelin: from inert membrane to perfusion pump. Neurochem Res 27: 1279–1292.
Ellison-Wright I, Bullmore E (2009). Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 108: 3–10.
First MB, Spitzer RL, Gibbon M, Williams JBW (2002a) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP). Biometrics Research, New York State Psychiatric Institute: New York, NY, USA.
First MB, Spitzer RL, Gibbon M, Williams JBW (2002b) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Biometrics Research, New York State Psychiatric Institute: New York, NY, USA.
Fitzsimmons J, Kubicki M, Smith K, Bushell G, Estepar RS, Westin CF et al (2009). Diffusion tractography of the fornix in schizophrenia. Schizophr Res 107: 39–46.
Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP et al (2003). Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry 8: 811–820.
Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA (2000). Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 68: 242–244.
Foong J, Symms MR, Barker GJ, Maier M, Miller DH, Ron MA (2002). Investigating regional white matter in schizophrenia using diffusion tensor imaging. Neuroreport 13: 333–336.
Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L et al (2008). Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry 165: 1024–1032.
Gasparotti R, Valsecchi P, Carletti F, Galluzzo A, Liserre R, Cesana B et al (2009). Reduced fractional anisotropy of corpus callosum in first-contact, antipsychotic drug-naive patients with schizophrenia. Schizophr Res 108: 41–48.
Gulani V, Webb AG, Duncan ID, Lauterbur PC (2001). Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med 45: 191–195.
Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD et al (2001). Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 98: 4746–4751.
Hall RC (1995). Global assessment of functioning. A modified scale. Psychosomatics 36: 267–275.
Haroutunian V, Katsel P, Dracheva S, Stewart DG, Davis KL (2007). Variations in oligodendrocyte-related gene expression across multiple cortical regions: implications for the pathophysiology of schizophrenia. Int J Neuropsychopharmacol 10: 565–573.
Highley JR, Esiri MM, McDonald B, Cortina-Borja M, Herron BM, Crow TJ (1999a). The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: a post-mortem study. Brain 122 (Pt 1): 99–110.
Highley JR, Esiri MM, McDonald B, Roberts HC, Walker MA, Crow TJ (1999b). The size and fiber composition of the anterior commissure with respect to gender and schizophrenia. Biol Psychiatry 45: 1120–1127.
Highley JR, Walker MA, Esiri MM, Crow TJ, Harrison PJ (2002). Asymmetry of the uncinate fasciculus: a post-mortem study of normal subjects and patients with schizophrenia. Cereb Cortex 12: 1218–1224.
Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M (2003). Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry 60: 585–594.
Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD (2002). Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res 27: 1193–1200.
Hof PR, Haroutunian V, Friedrich VL Jr, Byne W, Buitron C, Perl DP et al (2003). Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry 53: 1075–1085.
Jablensky A (2000). Epidemiology of schizophrenia: the global burden of disease and disability. Eur Arch Psychiatry Clin Neurosci 250: 274–285.
Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J (2012). Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp 34: 2747–2766.
Jones DK, Horsfield MA, Simmons A (1999). Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 42: 515–525.
Jones DK, Symms MR, Cercignani M, Howard RJ (2005). The effect of filter size on VBM analyses of DT-MRI data. Neuroimage 26: 546–554.
Jones DK (2004). The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med 51: 807–815.
Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD (2009). White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry 66: 562–569.
Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD (2008). Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry 63: 512–518.
Katsel P, Davis KL, Haroutunian V (2005). Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophr Res 79: 157–173.
Kay SR, Fiszbein A, Opler LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276.
Kito S, Jung J, Kobayashi T, Koga Y (2009). Fiber tracking of white matter integrity connecting the mediodorsal nucleus of the thalamus and the prefrontal cortex in schizophrenia: a diffusion tensor imaging study. Eur Psychiatry 24: 269–274.
Koch K, Wagner G, Dahnke R, Schachtzabel C, Schultz C, Roebel M et al (2010). Disrupted white matter integrity of corticopontine-cerebellar circuitry in schizophrenia. Eur Arch Psychiatry Clin Neurosci 260: 419–426.
Koch K, Wagner G, Schachtzabel C, Schultz CC, Gullmar D, Reichenbach JR et al (2011). Neural activation and radial diffusivity in schizophrenia: combined fMRI and diffusion tensor imaging study. Br J Psychiatry 198: 223–229.
Kong X, Ouyang X, Tao H, Liu H, Li L, Zhao J et al (2011). Complementary diffusion tensor imaging study of the corpus callosum in patients with first-episode and chronic schizophrenia. J Psychiatry Neurosci 36: 120–125.
Kubicki M, Styner M, Bouix S, Gerig G, Markant D, Smith K et al (2008). Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophr Res 106: 125–131.
Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE et al (2003). Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry 54: 1171–1180.
Kuroki N, Kubicki M, Nestor PG, Salisbury DF, Park HJ, Levitt JJ et al (2006). Fornix integrity and hippocampal volume in male schizophrenic patients. Biol Psychiatry 60: 22–31.
Kyriakopoulos M, Frangou S (2009). Recent diffusion tensor imaging findings in early stages of schizophrenia. Curr Opin Psychiatry 22: 168–176.
Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N et al (2001). Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13: 534–546.
Lee K, Yoshida T, Kubicki M, Bouix S, Westin CF, Kindlmann G et al (2009). Increased diffusivity in superior temporal gyrus in patients with schizophrenia: a Diffusion Tensor Imaging study. Schizophr Res 108: 33–40.
Leemans A, Jones DK (2009). The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 61: 1336–1349.
Leemans A, Jeurissen B, Sijbers J, Jones DK (2009). ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Presented at 17th Annual Meeting of Intl Soc Mag Reson Med (ISMRM), Hawaii, USA.
Levitt JJ, Kubicki M, Nestor PG, Ersner-Hershfield H, Westin CF, Alvarado JL et al (2010). A diffusion tensor imaging study of the anterior limb of the internal capsule in schizophrenia. Psychiatry Res 184: 143–150.
Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A (1999). Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry 56: 367–374.
McIntosh AM, Munoz Maniega S, Lymer GK, McKirdy J, Hall J, Sussmann JE et al (2008). White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry 64: 1088–1092.
Miyata J, Yamada M, Namiki C, Hirao K, Saze T, Fujiwara H et al (2010). Reduced white matter integrity as a neural correlate of social cognition deficits in schizophrenia. Schizophr Res 119: 232–239.
Molina V, Reig S, Sanz J, Palomo T, Benito C, Sanchez J et al (2005). Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr Res 80: 61–71.
Mori S, van Zijl P (2007a). Human white matter atlas. Am J Psychiatry 164: 1005.
Mori T, Ohnishi T, Hashimoto R, Nemoto K, Moriguchi Y, Noguchi H et al (2007b). Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Res 154: 133–145.
Murakami M, Takao H, Abe O, Yamasue H, Sasaki H, Gonoi W et al (2011). Cortical thickness, gray matter volume, and white matter anisotropy and diffusivity in schizophrenia. Neuroradiology 53: 859–866.
Nichols TE, Holmes AP (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25.
Okugawa G, Nobuhara K, Minami T, Takase K, Sugimoto T, Saito Y et al (2006). Neural disorganization in the superior cerebellar peduncle and cognitive abnormality in patients with schizophrenia: A diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry 30: 1408–1412.
Patel S, Mahon K, Wellington R, Zhang J, Chaplin W, Szeszko PR (2011). A meta-analysis of diffusion tensor imaging studies of the corpus callosum in schizophrenia. Schizophr Res 129: 149–155.
Perez-Iglesias R, Tordesillas-Gutierrez D, Barker GJ, McGuire PK, Roiz-Santianez R, Mata I et al (2010a). White matter defects in first episode psychosis patients: a voxelwise analysis of diffusion tensor imaging. Neuroimage 49: 199–204.
Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I et al (2010b). White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry 167: 451–458.
Peters BD, de Haan L, Dekker N, Blaas J, Becker HE, Dingemans PM et al (2008). White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology 58: 19–28.
Phillips OR, Nuechterlein KH, Clark KA, Hamilton LS, Asarnow RF, Hageman NS et al (2009). Fiber tractography reveals disruption of temporal lobe white matter tracts in schizophrenia. Schizophr Res 107: 30–38.
Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996). Diffusion tensor MR imaging of the human brain. Radiology 201: 637–648.
Price G, Bagary MS, Cercignani M, Altmann DR, Ron MA (2005). The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 76: 585–587.
Rotarska-Jagiela A, Oertel-Knoechel V, DeMartino F, van de Ven V, Formisano E, Roebroeck A et al (2009). Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res 174: 9–16.
Rotarska-Jagiela A, Schonmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DE (2008). The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage 39: 1522–1532.
Rowland LM, Spieker EA, Francis A, Barker PB, Carpenter WT, Buchanan RW (2009). White matter alterations in deficit schizophrenia. Neuropsychopharmacology 34: 1514–1522.
Scheepers FE, Gispen de Wied CC, Hulshoff Pol HE, Kahn RS (2001). Effect of clozapine on caudate nucleus volume in relation to symptoms of schizophrenia. Am J Psychiatry 158: 644–646.
Schlosser RG, Nenadic I, Wagner G, Gullmar D, von Consbruch K, Kohler S et al (2007). White matter abnormalities and brain activation in schizophrenia: a combined DTI and fMRI study. Schizophr Res 89: 1–11.
Seal ML, Yucel M, Fornito A, Wood SJ, Harrison BJ, Walterfang M et al (2008). Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr Res 101: 106–110.
Seok JH, Park HJ, Chun JW, Lee SK, Cho HS, Kwon JS et al (2007). White matter abnormalities associated with auditory hallucinations in schizophrenia: a combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Res 156: 93–104.
Shergill SS, Kanaan RA, Chitnis XA, O’Daly O, Jones DK, Frangou S et al (2007). A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry 164: 467–473.
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31: 1487–1505.
Smith SM, Nichols TE (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44: 83–98.
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17: 1429–1436.
Sun Z, Wang F, Cui L, Breeze J, Du X, Wang X et al (2003). Abnormal anterior cingulum in patients with schizophrenia: a diffusion tensor imaging study. Neuroreport 14: 1833–1836.
Sussmann JE, Lymer GK, McKirdy J, Moorhead TW, Munoz Maniega S, Job D et al (2009). White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord 11: 11–18.
Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S et al (2005). White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry 162: 602–605.
Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S et al (2008). Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology 33: 976–984.
Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB et al (2003). Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 362: 798–805.
Tournier JD, Mori S, Leemans A (2011). Diffusion tensor imaging and beyond. Magn Reson Med 65: 1532–1556.
Vos SB, Jones DK, Jeurissen B, Viergever MA, Leemans A (2012). The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. Neuroimage 59: 2208–2216.
Vos SB, Jones DK, Viergever MA, Leemans A (2011). Partial volume effect as a hidden covariate in DTI analyses. Neuroimage 55: 1566–1576.
Wang F, Sun Z, Cui L, Du X, Wang X, Zhang H et al (2004). Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry 161: 573–575.
Wang F, Sun Z, Du X, Wang X, Cong Z, Zhang H et al (2003). A diffusion tensor imaging study of middle and superior cerebellar peduncle in male patients with schizophrenia. Neurosci Lett 348: 135–138.
Wheeler-Kingshott CA, Cercignani M (2009). About ‘axial’ and ‘radial’ diffusivities. Magn Reson Med 61: 1255–1260.
Whitford TJ, Kubicki M, Schneiderman JS, O’Donnell LJ, King R, Alvarado JL et al (2010). Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry 68: 70–77.
Wilkins A, Majed H, Layfield R, Compston A, Chandran S (2003). Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci 23: 4967–4974.
Woodruff PW, McManus IC, David AS (1995). Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry 58: 457–461.
Zou LQ, Xie JX, Yuan HS, Pei XL, Dong WT, Liu PC (2008). Diffusion tensor imaging study of the anterior limb of internal capsules in neuroleptic-naive schizophrenia. Acad Radiol 15: 285–289.
Acknowledgements
We gratefully acknowledge the participants who generously gave their time and make this study possible, and Siemens for providing a Special Sequence WIP to enable DTI data acquisition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holleran, L., Ahmed, M., Anderson-Schmidt, H. et al. Altered Interhemispheric and Temporal Lobe White Matter Microstructural Organization in Severe Chronic Schizophrenia. Neuropsychopharmacol 39, 944–954 (2014). https://doi.org/10.1038/npp.2013.294
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.294
Keywords
This article is cited by
-
White and Gray Matter Abnormalities in Young Adult Females with Dependent Personality Disorder: A Diffusion-Tensor Imaging and Voxel-Based Morphometry Study
Brain Topography (2024)
-
Magnetic resonance diffusion tensor imaging in psychiatry: a narrative review of its potential role in diagnosis
Pharmacological Reports (2021)
-
Widespread Disrupted White Matter Microstructure in Autism Spectrum Disorders
Journal of Autism and Developmental Disorders (2019)
-
Cannabinoids and glial cells: possible mechanism to understand schizophrenia
European Archives of Psychiatry and Clinical Neuroscience (2018)
-
Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia
Translational Psychiatry (2017)