Abstract
Recent in vitro results suggest that cocaine may exert direct and/or indirect allosteric enhancing actions at dopamine (DA) D2 receptors (D2Rs). In the present paper we tested the hypothesis that cocaine in vivo can enhance the effects of the D2-likeR agonist quinpirole in rats by using microdialysis and pharmacological behavioral studies. Furthermore, in vitro D2-likeR binding characteristics and Gαi/o-protein coupling, in the absence and in the presence of cocaine, have been investigated in rat striatal membranes. Intra-nucleus accumbens perfusion of the D2-likeR agonist quinpirole (10 μM) reduced local dialysate glutamate levels, whereas cocaine (10 and 100 nM) was ineffective. At a low concentration (100 nM), cocaine significantly enhanced quinpirole-induced reduction of accumbal extracellular glutamate levels. The behavioral experiments showed that cocaine (0.625 mg/kg), but not the DA uptake blocker GBR 12783 (1.25 mg/kg), enhanced quinpirole (1 mg/kg)-induced hyperlocomotion. Finally, cocaine (100 nM), but not GBR 12783 (200 nM), produced a small, but significant increase in the efficacy of DA to stimulate binding of GTPγS to striatal D2-likeRs, whereas the D2-likeR binding characteristics were unchanged in striatal membranes by cocaine in the nM range. The significant increase in the maximal response to DA-stimulated GTPγS binding to D2-likeRs by 100 nM cocaine remained in the presence of GBR 12783. The observed cocaine-induced enhancement of the Gαi/o-protein coupling of D2Rs may be in part because of allosteric direct and/or indirect enhancing effects of cocaine at these receptors. These novel actions of cocaine may have relevance for understanding the actions of cocaine upon accumbal DA, and/or glutamate transmission and thus its rewarding as well as relapsing effects.
Similar content being viewed by others
INTRODUCTION
It is well known that cocaine blocks the dopamine (DA) transporter in the plasma membrane of striatal DA nerve terminal networks. This mechanism leads to a marked increase of DA transmission, especially volume transmission (Agnati et al, 2006; Fuxe et al, 2007; Rice and Cragg, 2008; Fuxe et al, 2009), and is thought to underlie the rewarding/reinforcing actions of cocaine in humans that lead to drug abuse and addiction (Di Chiara and Imperato, 1988; Koob and Bloom, 1988; Kalivas and Volkow, 2005; De Mei et al, 2009). Besides this action, recent in vitro results suggest that cocaine may exert enhancing allosteric actions at DA D2 receptors (D2Rs) and/or associated proteins. In particular, in cell lines lacking the DA transporter, cocaine was reported to modulate D2R agonist recognition, G-protein coupling, and signaling (Marcellino et al, 2009, 2010). In addition, cocaine at a concentration (10 nM) determined not to inhibit DA reuptake, significantly enhanced the ability of the D2-likeR agonist quinpirole to reduce K+-evoked [3H]DA efflux from rat striatal synaptosomes (Ferraro et al, 2010). Finally, to further support the existence of possible direct and/indirect allosteric actions of cocaine at D2Rs, a recent study demonstrated that 150 nM cocaine produced a significant increase of membrane associated D2R immunoreactivity in CHO cell lines lacking the DA transporter (Genedani et al, 2010).
The above findings are of particular relevance in view of the well-established role of D2Rs in the abuse-related effects of cocaine (Self, 2010). Stimulation of D2Rs in the ventral striatum (nucleus accumbens) and the dorsal striatum is involved in mediating rewarding or relapsing behavioral responses of cocaine, respectively (Spealman et al, 1999; Di Chiara et al, 2004; Filip et al, 2006). Thus, the direct allosteric enhancing action of cocaine at striatal D2Rs and/or indirect actions via associated proteins and/or discrete lipids may have relevance for understanding its activity on striatal DA transmission and consequently its rewarding as well as relapsing actions. However, at present, there are no existing studies indicating that cocaine can also exert such actions in vivo. To evaluate the possibility that cocaine can enhance the effects of the D2-likeR agonist quinpirole in vivo we employed pharmacological behavioral analyses and microdialysis in awake freely moving rats. Furthermore, in view of the results obtained in vivo, the Gαi/o-protein coupling of the D2-likeRs and its agonist binding characteristics were also investigated in rat striatal membranes in the absence and in the presence of nanomolar concentrations of cocaine.
MATERIALS AND METHODS
In Vivo Microdialysis
Male Sprague–Dawley rats (Harlan Italy S. r.l.; 300–320 g) were used. The animals were housed in a temperature and relative humidity controlled environment with a regular 12-hour light/dark cycle (lights on at 0600 hours) and had free access to food and water. The animals were allowed to adapt to the environment for at least 1 week before experimental procedures. Experiments were carried out in strict accordance with the European Communities Council Directive (86/609/EEC) and the Guidelines released by the Italian Ministry of Health (D.L. 116/92) and (D.L. 111/94-B). All efforts were made to minimize the number of animals used and their suffering.
Surgery
The animals, kept under isoflurane anesthesia (1.5% mixture of halothane and air), were mounted in a stereotaxic frame with the upper incisor bar set at −2.5 mm below the interaural line. After exposing the skull and drilling a hole, a microdialysis probe of concentric design (CMA 12; MW cutoff 20 000 Da; outer diameter 0.5 mm; length of dialysing membrane 1 mm) was implanted into the right or the left nucleus accumbens (stereotaxic coordinates: A: +1.3; L: ±1.4; V: −7.5) (Paxinos and Watson, 1986). Following the implantation, the probe was permanently secured to the skull with methacrylic cement and 36 h later the experiments were performed.
Experimental protocol
On the day of the experiment, the probe was continuously perfused with Ringer solution (in mM: Na+ 147; K+ 4; Ca++ 1.4; Cl− 156; glucose 2.7) at a constant flow rate (2 μl/min) using a CMA 100 microinfusion pump. The collection of perfusate samples commenced 300 min after the onset of perfusion to achieve stable dialysate glutamate levels and perfusates were collected every 20 min. The D2-likeR agonist quinpirole hydrochloride (10 μM; Tocris, Ellisville, MO, USA) and cocaine hydrochloride (10 or 100 nM; Sigma-Aldrich, St Louis, MO, USA), alone and in combination, were locally perfused by reverse dialysis for 60 min after three stable baseline glutamate levels had been reached. This medium was then replaced with the original perfusate and an additional three samples were collected (60 min). The experiments were also performed in the presence of the DA uptake blocker GBR 12783 (1 μM; Bonnet and Costentin, 1986), added to the perfusion medium 2 h before the sample collection. At the end of each experiment, the brain was removed from the skull and the position of the probe was carefully verified in 30 μm-thick coronal cryostat sections. Only those animals in which the probe was correctly located were included in this study.
In a final set of microdialysis experiments to study the spread of cocaine, N-methyl-[3H]-cocaine (1 mCi/ml; specific activity: 80 Ci/mmol; American Radiolabeled Chemicals, St Louis, USA) was perfused at 100 nM concentration into the nucleus accumbens (60 min; flow rate 2 μl/min). After this period, the animals were killed; the nucleus accumbens and the ipsilateral prefrontal cortex were rapidly removed and then solubilized in 2 ml of NaOH (1 M). The radioactivity of each sample was determined by liquid scintillation spectrometry (LS1800 Beckman).
Glutamate analysis
Endogenous glutamate levels were quantified using a HPLC/fluorimetric detection, including precolumn derivatization with o-phtaldialdehyde reagent and a Chromsep 5 (C18) column as previously described (Ferraro et al, 1998). The mobile phase consisted of 0.1 M sodium acetate, 10% methanol, and 2.5% tetrahydrofurane, pH 6.5. The limit of detection for glutamate was 30 fmol/sample.
Data analysis
Data from individual time points are reported as percentages of the mean of the three basal samples before treatment. The data were calculated as mean±SEM and only the significance with regard to the peak effects (maximal responses) is shown in the figures. In addition, the area created by the curve, which mainly reflects the duration of the effect under the experimental period, was calculated for each animal. The area values (overall effects) were calculated as percentages of changes in basal value over time (Δ basal percentage × time) by using the trapezoidal rule. The statistical analysis was carried out by analysis of variance (ANOVA) followed by the Newman–Keuls test for multiple comparisons. All comparisons were made with an experiment wise type I error rate (α) set at 0.05.
Behavioral Experiments
Male Wistar rats (Charles River, Germany; 200–220 g) were used. The animals were housed in a colony room maintained at temperature and relative humidity controlled with a regular 12-hour light/dark cycle (lights on at 0600 hours). Rodent chow and water were available ad libitum. All the experiments were conducted during the light phase of the light-dark cycle (between 0800 and 1400 hours), in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the approval of the Local Bioethics Commission (and compliant with the Polish Law, August 21, 1997). All the efforts were made to minimize animal suffering and to reduce the number of rats used.
Locomotor activity measurements
Locomotor activity was recorded individually for each animal in Opto-Varimex cages (Columbus Instruments, Columbus, OH, USA) linked on-line to a compatible IBM-PC. Each chamber (43 cm × 43 cm × 21 cm), equipped with a 220-lx house light, was made of transparent acrylic plastic (all six sides) and was housed in a light- and soundproof wooden cubicle. The corner brackets were made of stainless steel. Each cage was surrounded by a 15 × 15 array of photocell beams located 3 cm from the floor surface as reported previously (Frankowska et al, 2009; Zaniewska et al, 2010). Interruptions of the photobeams resulted in the measurement of horizontal locomotor activity, defined as a distance traveled, expressed in centimeter. Rats were habituated in the experimental cages for 2 days (120 min/day) and for 60 min before testing; afterwards, the animals were taken out, injected with the appropriate drug, and put back into the cages. Animals were pretreated with either vehicle or cocaine hydrochloride (0.625 mg/kg, i.p.; Sigma Aldrich, USA) given 5 min before vehicle or quinpirole hydrochloride (0.5–1 mg/kg, i.p.) injection. Other groups of rats were pretreated with either vehicle or GBR 12783 (1.25 mg/kg, i.p.; Tocris, UK) given 30 min before vehicle or quinpirole hydrochloride (0.5 mg/kg, i.p.) injection. Measurements of locomotor activity began immediately after injection of quinpirole or its vehicle and were recorded for a total of 120 min. Six to ten animals per group were used.
Drugs were injected in a volume of 1 ml/kg. The doses of quinpirole and its pretreatment time are in agreement with the previously published studies (Marcellino et al, 2008), whereas doses of cocaine (0.625 mg/kg) and GBR 12783 (1.25 mg/kg) were based on our detailed dose–response curve investigations on locomotor activity. Namely, in combination behavioral experiments with quinpirole we have chosen the behaviorally inactive doses of GBR 12783 and cocaine, ie, subthreshold doses that have not altered the motor functions of the rats by themselves under equivalent locomotor conditions. In other words, being inactive (as compared with vehicle controls) under the same experimental procedure the chosen subthreshold doses of GBR 12783 or of cocaine have produced an equivalent locomotor activity level.
Behavioral data analysis
The data are expressed as total horizontal locomotor activity means (±SEM) for the 120-min observation period. The data were analyzed using a two-way ANOVA for the factors of pretreatment (vehicle and 0.625 mg/kg. of cocaine, or vehicle and 1.25 mg/kg of GBR 12783), treatment (vehicle and different doses of quinpirole), and the pretreatment × treatment interaction, followed by post hoc Newman–Keuls test, used to evaluate the treatment group effect. All comparisons were made with an experiment wise type I error rate (α) set at 0.05.
Radioligand-Binding Experiments
Membrane preparation
Male Sprague–Dawley rat (Charles-River, Germany) striatal membranes were prepared for their use in radioligand-binding experiments. Rats were killed by decapitation and the brains were removed and cooled for 2 min in ice-cold saline. The dorsal striatum from each animal was dissected out and immediately frozen on dry ice and later stored at −80 °C until use. Dorsal striatal tissue was placed in 10 ml of preparation buffer (PB; 50 mM Tris–HCl, pH 7.4, containing NaCl 100 mM, MgCl2 7 mM and EDTA 1 mM) containing a cocktail of protease inhibitors (Roche Diagnostics, Mannheim, Germany). The tissue was homogenized using a basic IKA T18 mechanical tissue homogenizer (IKA Werke, Staufen, Germany) and centrifuged at 40 000 g for 40 min at 4 °C. The pelleted membranes were resuspended and homogenized in the same PB and centrifuged an additional three times. The protein concentration was determined for the pelleted membranes by the BCA Protein Assay Kit (Pierce, Rockford, IL, USA) using bovine serum albumin (BSA) dilutions to construct a standard curve. Pelleted membranes were resuspended to a concentration of 2 mg/ml and aliquots were stored at −80 °C.
[3H]raclopride binding assay
The D2-likeR antagonist [3H]raclopride (2.0–3.0 nM;. specific activity 82.8 Ci/mmol, Perkin-Elmer Life Sciences, USA) binding was displaced by either DA (0.1 nM–1 mM; Sigma Aldrich, St Louis, USA) or quinpirole hydrochloride (0.03 nM–0.3 mM) to determine agonist affinities from the competition curves obtained. Experiments were performed using striatal rat membranes (120 μg protein/ml) and were incubated for 90 min at 30 °C in incubation buffer (IB; 50 mM Tris-HCl pH 7.4, containing: NaCl, 100 mM; MgCl2, 7 mM; EDTA, 1 mM, and DTT, 1 mM) in the absence or presence (1–100 nM) of cocaine hydrochloride. The incubation was terminated by rapid filtration through GF/C filters using a Brandel cell harvester with three washes of 5 ml of ice-cold wash buffer (WB; 50 mM Tris-HCl pH 7.4). Nonspecific binding was defined by the binding in the presence 10 μM (+)-butaclamol (Sigma Aldrich, USA) a D2-likeR antagonist.
[35S]GTPγS binding assay
Binding of [35S]GTPγS ([35S]-guanosine5-([γ]-thio)triphosphate, specific activity 1250 Ci/mmol; PerkinElmer Life Sciences, USA) to membranes was carried out in IB as described by Rinken et al (1999). In brief, membranes (100 μg/ml) were incubated with 0.08–0.12 nM [35S]GTPγS, 50 μM GDP (guanosine 5′-diphosphate sodium salt; Sigma Aldrich, USA), and increasing concentrations of DA (1 nM–1 mM) for 90 min at 30 °C in the absence or presence (1–100 nM) of cocaine hydrochloride, 200 nM GBR 12783 (Tocris, UK), 100 nM cocaine plus 200 nM GBR 12783, or 100 nM cocaine plus 1 mM raclopride (Sigma Aldrich, USA). The reaction was terminated by rapid filtration through GF/C filters using a Brandel cell harvester and with three washes of 5 ml of ice-cold WB. Nonspecific binding was defined as the binding in the presence of 10 μM (+)-butaclamol.
In vitro data analysis
All binding data were analyzed using the commercial program Graph Pad PRISM 4.0 (Graph Pad software, San Diego, USA). For statistical evaluation of the biochemical data, a one-way of ANOVA was used on the pKi values obtained from independent experiments and group differences were measured by a post hoc Dunnett's multiple comparison test. In the case of two groups Student's paired t-test was used.
RESULTS
In Vivo Microdialysis
Endogenous extracellular glutamate levels in the nucleus accumbens of the awake rat
Basal extracellular glutamate levels (not normalized for probe recovery) in the nucleus accumbens of the awake rat averaged 7.4±0.03 pmol/sample (mean±SEM; n=22). These levels were stable over the duration of the experiments (180 min).
Effects of quinpirole and cocaine, alone or in combination, on extracellular glutamate levels in the nucleus accumbens of the awake rat
As shown in Figure 1, intra-nucleus accumbens perfusion of the D2-likeR agonist quinpirole (10 μM) significantly reduced basal extracellular glutamate levels in the nucleus accumbens of the awake rat. These levels significantly decreased 40 min after initiating quinpirole perfusion and gradually returned to basal levels following the removal of quinpirole from the perfusion solution.
Effect of intra-nucleus accumbens perfusion with the dopamine D2-like receptor agonist quinpirole (Quin) or cocaine (10 and 100 nM) on extracellular glutamate levels in the nucleus accumbens of the awake rat. The substances were perfused for 60 min (bar). The results are expressed as percentage of the mean of the three basal values (193±9 nM) before treatment. The areas under the curves, which mainly represent the integrated time–response curve of the overall effects, calculated as percent changes in basal value over time (±basal % × time) by using the trapezoidal rule are reported on the right side. Each point represents the mean±SEM of 5–7 animals. Control rats were perfused with normal Ringer perfusion medium throughout the experiment. *P<0.05 **P<0.01 significantly different from all the other groups according to analysis of variance followed by Newman–Keuls test for multiple comparisons.
On the contrary, basal extracellular glutamate levels were not significantly altered by the intra-nucleus accumbens perfusion of 10 or 100 nM cocaine (Figure 1).
Interestingly, when 100 nM cocaine was perfused in the nucleus accumbens in combination with quinpirole (10 μM), the D2-likeR agonist-induced inhibition of extracellular glutamate levels was significantly amplified (Figure 2). This effect was not produced by the lower concentration of cocaine (10 nM) tested (Figure 2). The effect of 100 nM cocaine was significant when evaluating either the time-course or the areas under the curves, which mainly represent the integrated time–response curve of the overall effects, calculated as percent changes in the basal value over time (±basal % × time) by using the trapezoidal rule.
Effect of intra-nucleus accumbens perfusion with the dopamine D2-like receptor agonist quinpirole (Quin) in combination with cocaine 10 nM (a) or 100 nM (b) on extracellular glutamate levels in the nucleus accumbens of the awake rat. The substances were simultaneously perfused for 60 min (bar). The results are expressed as percentage of the mean of the three basal values (179±8 nM) before treatment. The areas under the curves, which mainly represent the integrated time–response curve of the overall effects, calculated as percent changes in basal value over time (±basal % × time) by using the trapezoidal rule are reported on the right side. Each point represents the mean±SEM of 6–7 animals. Control rats were perfused with normal Ringer perfusion medium throughout the experiment. Panel A: *P<0.05; **P<0.01 significantly different from control as well as cocaine alone groups; Panel B: *P<0.05; **P<0.01 significantly different from control as well as cocaine alone groups. °P<0.01 significantly different from Quin group according to analysis of variance followed by Newman–Keuls test for multiple comparisons.
To determine whether the significant amplification of D2-likeR agonist-induced inhibition of extracellular glutamate levels by cocaine was due in part to the ability of cocaine to block the DA transporter, similar microdialysis experiments were performed in the presence of a saturating concentration of GBR 12783, a selective inhibitor of DA transporter (Bonnet and Costentin, 1986). The effect of cocaine (100 nM) on quinpirole (10 μM)-induced inhibition of extracellular glutamate levels was also observed in presence of GBR 12783 (1 μM) added to the perfusion medium 2 h before the sample collection period (Figure 3). It is worth noting that under these experimental conditions, the effects of quinpirole, alone or in presence of cocaine, are lower than those observed in the absence of GBR 12783. However, these reductions do not reach statistical significance. Thus, under the conditions of a complete DA transporter blockade by GBR 12783, 100 nM cocaine was still able to significantly enhance quinpirole-induced inhibition of extracellular glutamate levels (Figure 3). When added to the perfusion medium, GBR 12783 (1 μM) induced a slight, nonsignificant, reduction in nucleus accumbens extracellular glutamate levels (89±4% of basal values).
Effect of intra-nucleus accumbens perfusion with quinpirole (Quin) and cocaine 100 nM, in presence of the DA uptake blocker GBR 12783 (1 μM) on extracellular glutamate levels in the nucleus accumbens of the awake rat. Quin and cocaine were simultaneously perfused for 60 min (black bar); GBR 12783 was added to the perfusion medium 2 h before the sample collection period (open bar). The results are expressed as percentage of the mean of the three basal values (168±11 nM) before treatment. The areas under the curves, which mainly represent the integrated time–response curve of the overall effects, calculated as percent changes in basal value over time (±basal % × time) by using the trapezoidal rule are reported on the right side. Each point represents the mean±SEM of 6–7 animals. Control rats were perfused with normal Ringer perfusion medium throughout the experiment. *P<0.05; **P<0.01 significantly different from control as well as cocaine alone groups. °P<0.01 significantly different from Quin group according to analysis of variance followed by Newman–Keuls test for multiple comparisons.
Intra-accumbens perfusion of N-methyl-[3H]-cocaine
In a final set of microdialysis experiments, the possible diffusion of cocaine to the prefrontal cortex after its intra-accumbens perfusion was evaluated. To this purpose N-methyl-[3H]-cocaine was perfused at a concentration of 100 nM into the nucleus accumbens (60 min; flow rate 2 μl/min). The radioactivity of tissue lysates from the nucleus accumbens and ipsilateral prefrontal cortex was measured and concentrations of radiolabeled cocaine were determined to only reach 0.5±0.07 nM in the prefrontal cortex in comparison with 8±0.5 nM in the nucleus accumbens.
Behavioral experiments
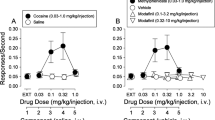
In view of the above results and in order to determine whether the amplification of D2-likeR agonist-induced inhibition of extracellular glutamate levels by cocaine could be associated with behavioral consequences, the effects of cocaine on quinpirole-induced hyperlocomotion have been assessed. A main overall effect of pretreatment (F1,54=5.59, P<0.01), treatment (F2,54=4.83, P<0.05), and pretreatment x treatment interaction (F2,54=2.39, P<0.05) was observed in groups of rats pretreated with cocaine (0.625 mg/kg). Given in combination with vehicle, cocaine did not alter locomotor activity, whereas quinpirole (0.5 and 1 mg/kg) significantly (∼100% and 180%, respectively) enhanced basal locomotor activity compared with vehicle + vehicle group. Cocaine at a dose of 0.625 mg/kg administered in combination with 0.5 mg/kg of quinpirole nonsignificantly increased (∼170%) the locomotor response to acute quinpirole (0.5 mg/kg), whereas a significant (P<0.05) enhancement was observed for a higher dose of quinpirole (1 mg/kg) following pretreatment with cocaine (0.625 mg/kg) before quinpirole (1 mg/kg; Figure 4).
Effects of cocaine (Coc; 0.625 mg/kg) on the basal or quinpirole (Quin; 0.5–1 mg/kg) -stimulated locomotor activity in rats. Total (120 min) horizontal locomotor activity means (±SEM) of data from 10 rats per group after administration of vehicle (Veh) or Coc followed by injection of Veh or Quin. *P<0.05, **P<0.01 vs Veh+Veh group; ^P<0.05 vs Veh+Quin 1 group according a two-way analysis of variance followed by Newman–Keuls test for multiple comparisons.
A main effect of treatment (F1,21=14.69, P<0.001), but not of pretreatment (F1,21=0.61) and of pretreatment × mμ treatment (F1,21=0.38), was observed in group of rats pretreated with GBR 12873 (1.25 mg/kg). GBR 12783 at the dose of 1.25 mg/kg did not change either the rats' basal locomotor activity or quinpirole-induced hyperactivity (Figure 5).
Effects of GBR 12783 (GBR; 1.25 mg/kg) on the basal or quinpirole (Quin; 0.5 mg/kg) -stimulated locomotor activity in rats. Total (120 min) horizontal locomotor activity means (±SEM) of data from six to eight rats per group after administration of vehicle (Veh) or GBR followed by injection of Veh or Quin. *P<0.05 vs Veh+Veh group; #P<0.05 vs Veh+GBR 1.25 group according a two-way analysis of variance followed by Newman–Keuls test for multiple comparisons.
In Vitro
D2-likeR binding using [3h]raclopride
Nanomolar concentrations of cocaine (1–100 nM) failed to modulate quinpirole binding characteristics of D2-likeRs in competition experiments using the D2-likeR antagonist [3H]raclopride (2.5 nM), as radioligand and increasing concentrations of either quinpirole (Figure 6a) or DA (Figure 6b) in the absence or presence of cocaine (Figure 6; 100 nM cocaine, the maximum concentration tested). As shown in Figure 6, biphasic competition curves were observed for DA and quinpirole. Cocaine at concentrations of 1 and 10 nM failed to produce any effect on DA receptor agonist quinpirole and DA affinity at D2-likeRs (data not shown). Thus, no significant changes in the high or low affinity dissociation constants for agonist binding or in the proportion of high-affinity agonist binding sites of D2-likeRs were produced by 100 nM cocaine (Table 1) or any of lower concentrations of cocaine tested (data not shown).
Absence of effects of cocaine (100 nM) on D2-like receptor agonist quinpirole and DA binding. Competition experiments of the DA D2-like receptor antagonist [3H]raclopride (2.5 nM) vs increasing concentrations of the D2-like receptor agonist quinpirole (in a) or DA (in b) in rat striatal membranes in the presence (○—in a; □—in b) or absence (•—in a; ▪—in b) of cocaine (100 nM). Values (means±SEM) are expressed as % of specific binding to the sample in absence of ligand (control) from three to six independent experiments performed in duplicate.
[35S]GTPγS binding
[35S]GTPγS-binding experiments were performed in rat striatal membranes using increasing concentrations of DA in the presence or absence of nanomolar concentrations of cocaine (0.1, 1, 10, and 100 nM). At 100 nM, cocaine produced a small, but significant increase in the maximal response to DA-stimulated GTPγS binding to D2-likeRs (Figure 7a) without producing any significant effect in its potency as seen from the similar EC50 values in control and 100 nM cocaine-treated membranes presented in Table 2. On the contrary, at lower nM concentrations (0.1, 1, and 10 nM), cocaine failed to produce any significant effect on the maximal DA-stimulated binding of GTPγS to D2-likeRs and the EC50 values of DA were similar in the absence or presence of cocaine also at these lower concentrations (data not shown).
Effect of cocaine (100 nM) and GBR 12783 (200 nM) on dopamine-stimulated GTPγS binding to dopamine D2-like receptors. Binding of [35S]GTPγS (0.08–0.12) to dorsal striatal rat membranes (100 μg/ml) was measured after incubation with increasing concentrations of dopamine in the presence (○) or absence (•) of cocaine (a), the presence (○) or absence (•) of GBR 12783 (b), or the presence (○) or absence (•) of cocaine plus GBR 12783 (c). Raclopride (1 mM) in the presence of 100 nM cocaine (▪) prevented dopamine-stimulated GTPγS binding to dopamine D2-like receptors (c). Values are expressed as % of specific binding to the sample in the absence of dopamine (control) in which the basal binding activity was similar in the treatment and respective control groups. Data are presented as the means±SEM from three to six independent experiments performed in duplicate. Cocaine 100 nM in the presence or absence of GBR12783 significantly increased the Bmax value compared with control (Student's paired t-test; *P<0.05, **P<0.01).
To rule out the possibility that the significant increase of maximal response to DA-stimulated GTPγS binding produced by 100 nM cocaine was due to DA transporter blockade, [35S]GTPγS-binding experiments were performed using instead increasing concentrations of DA in the presence of GBR 12783. A concentration of GBR 12783 (200 nM) that has been demonstrated to fully block DA reuptake in rat striatal synapotosomal preparations from rat (Ferraro et al, 2010) produced no increase in the maximal response of DA-stimulated GTPγS accumulation in rat striatal membrane preparations (Figure 7b). In addition, the significant increase in the maximal response to DA-stimulated GTPγS binding to D2-likeRs by 100 nM cocaine remained in the presence of DA transporter blockade by GBR 12783 (Figure 7c). Together these results suggest that the effect produced by 100 nM cocaine was independent of DA transporter blockade.
DISCUSSION
Recent findings in cell lines devoid of the DA transporter indicate that cocaine in low to modest concentrations can alter D2R recognition, G-protein coupling, signaling, and trafficking (Marcellino et al, 2010; Genedani et al, 2010). The mechanism may involve, inter alia, direct allosteric enhancing actions of cocaine on D2Rs and/or indirect actions via associated proteins and/or discrete lipids. This hypothesis is further supported by the recent demonstration that cocaine (10 nM) in vitro significantly enhanced the ability of the D2-likeR agonist quinpirole to reduce K+-evoked [3H]DA efflux from rat striatal synaptosomes (Ferraro et al, 2010). This effect was observed in the absence of cocaine-induced blockade of the DA transporter and also when DA re-uptake was blocked by the addition of a saturating concentration (200 nM) of the DA uptake blocker GBR 12783 (Ferraro et al, 2010). Thus, striatal (dorsal striatum and nucleus accumbens) DA transmission may in low nanomolar concentrations of cocaine be primarily affected by a novel action of cocaine at D2Rs.
In the current paper, we show for the first time the possible allosteric enhancing actions of cocaine at D2Rs in live animals determining the effects of nanomolar concentrations of cocaine on D2-likeR inhibition of glutamate release. Specifically, microdialysis has been performed in the nucleus accumbens, a key brain area for the acute rewarding effects of cocaine (Self, 2010). Basal in vivo extracellular glutamate is derived from both neuronal and glial sources, and from vesicular or nonvesicular pools (Baker et al, 2002). Neuronal glutamate levels in the nucleus accumbens are mainly derived from the afferent terminals of axons originating from prefrontal cortex, hippocampus, amygdala and periventricular thalamus (Kalivas and Duffy, 1997) and two major mechanisms depending on D2R stimulation have been identified to control accumbal glutamate release. As evidenced by ultrastructural immunocytochemistry (Pickel et al, 2006), D2-likeRs in the nucleus accumbens exist, among others, on glutamate terminals forming synapses in this brain area in the rat. Functional study suggest that through activation of such presynaptically localized D2 heteroreceptors on striatal glutamate axons D2-likeRs DA may inhibit the release of glutamate in the nucleus accumbens from the above-mentioned limbic projections (Kalivas and Duffy, 1997, Tanganelli et al, 2004). However, the major D2R mechanism in control of neuronal glutamate release (see Tanganelli et al, 2004) is located at the medium spiny GABA neurons and involves D2-likeR-induced synthesis and release of endocannabinoids that activate inhibitory CB1 receptors localized on the glutamate terminals through a retrograde signaling feed-back that involves volume transmission (Giuffrida et al, 1999; Centonze et al, 2001; Kreitzer and Malenka, 2005; Yin and Lovinger, 2006; Uchigashima et al, 2007; Martire et al, 2011). D2-likeRs are known to produce phospholipase C activation (Hernandez-Lopez et al, 2000), which leads to increases in synthesis and release of anandamide (Giuffrida et al, 1999). It should also be considered that cocaine may via such actions produce a D2-likeR dependent activation of the synthesis, release, and extracellular diffusion of endocannabinoids leading to enhancement of CB1 signaling and glutamate release inhibition (Giuffrida et al, 1999; Martire et al, 2011). It is certainly also possible that cocaine may modulate CB1 receptor function on striatal glutamate terminals via similar types of actions. Although no data are available at present, modulation of multiple signals seem possible.
In line with a previous observation (Kalivas and Duffy, 1997), accumbal quinpirole (10 μM) perfusion in the current study reduced basal local dialysate glutamate levels in rats. Although these findings are consistent with the localization of accumbal D2-likeRs on local glutamate terminals (see above), they are quite surprising considering that the extracellular glutamate levels in the nucleus accumbens are mainly sustained by nonvesicular glutamate release (Baker et al, 2002). In line with this view, several studies reported that the basal levels of extracellular glutamate in the nucleus accumbens are only modestly reduced or unaffected by tetrodotoxin perfusion or by the depletion of extracellular Ca++ levels (Baker et al, 2002; Saulskaya and Mikhailova, 2002; Saulskaya and Soloviova, 2004; LaLumiere and Kalivas, 2008). Therefore, it seems unlikely that inhibition of synaptic release of glutamate is the main mechanism responsible for the reported extracellular glutamate reduction during quinpirole perfusion. Consequently, it appears that the regulation of glutamate levels could also occur from any number of coincident cellular actions that would alter glutamate glial release or uptake. In this context, there is evidence that under resting conditions, DA may modulate glutamate uptake in the striatum (Nieoullon et al, 1982; Kerkerian et al, 1987; Schneider et al, 1998), the most ventral part of which is formed by the nucleus accumbens. Thus, a temporal enhancement of glutamate uptake could explain the quinpirole-induced decrease in extracellular glutamate levels observed in the present study. Interestingly, Saulskaya and Mikhailova (2002) demonstrated that the intra-accumbal administration of the D2/D3 DA receptor antagonist raclopride completely prevented the feeding-induced decrease in local basal extracellular glutamate levels. Thus, the authors concluded that these data indicate that DA input to the nucleus accumbens via D2/D3 receptors is involved in regulation of glutamate extracellular levels. Taken together, the above results suggest that transporters for glutamate could be targets for a dopaminergic-mediated regulation in the nucleus accumbens. In fact, DA transporter–D2 receptor interactions have been demonstrated and found to be disrupted in schizophrenia (Lee et al, 2009). This mechanism, combined with an albeit modest reduction of vesicular glutamate release from glutamate terminals, which cannot be excluded, could be responsible for the observed D2-likeR-mediated decrease in basal glutamate levels. Finally, the possible involvement of the cystine–glutamate exchange, which provides the primary source of extracellular, nonvesicular glutamate (Baker et al, 2002; Xi et al, 2002) cannot be ruled out and further studies are required to address this issue more specifically.
In contrast to quinpirole, intra-nucleus accumbens perfusion with cocaine (10 and 100 nM) did not modify extracellular glutamate levels. This observation is in line with the results that these concentrations of cocaine are below the IC50 value of cocaine to inhibit the DA transporter (Woodward et al, 1995) and do not significantly alter extracellular striatal DA levels in our previous experiments (Ferraro et al, 2010). However, when cocaine (100 nM) was perfused in the nucleus accumbens in combination with quinpirole, a significantly more pronounced decrease in basal glutamate levels was observed. This effect was also observed when DA re-uptake was blocked by the addition of GBR 12783; thus, suggesting that nucleus accumbens glutamate transmission may in low nanomolar concentrations of cocaine be primarily affected by a novel action of cocaine at D2Rs. Intra-nucleus accumbens perfusion with GBR 12783 by itself induced a slight reduction in extracellular glutamate levels. This finding in consistent with previous results that demonstrated increased nucleus accumbens DA levels following local perfusion of amphetamine that lead to a reduction of local extracellular glutamate levels (Kalivas and Duffy, 1997). The GBR 12783-induced reduction, although nonsignificant, could explain why in the presence of the DA uptake inhibitor the effects of quinpirole alone, or in presence of cocaine, are lower than those observed in the absence of GBR 12783. These in vivo results also fit our previous in vitro results demonstrating that cocaine at concentrations below its ability to affect the DA transporter increased the ability of quinpirole to reduce the K+-evoked [3H]DA efflux from rat striatal (containing the nucleus accumbens) synaptosomes (Ferraro et al, 2010). Furthermore, the possibility that cocaine, via stimulation of other brain areas, exerted modulatory actions of accumbal glutamate release seems to be unlikely as the concentration of radiolabeled N-methyl-cocaine that spread to the prefrontal cortex following its intra-accumbens perfusion was too low (0.5 nM) to affect the activity of the prefronto-accumbens glutamate projections. Thus, whatever the origin of basal glutamate levels, the enhancing actions of cocaine on D2-likeR agonist-induced inhibition of accumbens glutamate release are likely exerted at the D2-likeR localized in the nucleus accumbens. However, as a previous in vitro study (Ferraro et al, 2010) demonstrated effects of cocaine at 10 nM concentration, the possibility that a biologically effective concentration of cocaine may have reached other adjacent areas projecting to the accumbens that might influence glutamate release (for example GABAergic inputs from the pallidum) cannot be ruled out.
The apparent discrepancy between the concentrations of cocaine found to be active in enhancing quinpirole-induced reduction of K+-evoked [3H]DA in striatal synaptosomes (10 nM; Ferraro et al, 2010) and of extracellular glutamate levels in the present study (100 nM) can be explained by the different methodological approach used. In fact, it is well known that the fundamental principle that governs microdialysis is diffusion. In its most common application, the removal of substances from the extracellular space takes place along a diffusion gradient from the extracellular space to the probe. This gradient is established by the continuous perfusion of the probe with a medium containing less concentration of the substances to be measured. In microdialysis experiments, the measured levels of the dialysate chemical substances correspond to their absolute interstitial concentrations only if complete equilibration has occurred over the dialysis membrane. The degree of equilibration for a permeable substance (relative recovery) is mainly dependent on the perfusion rate, the length of the dialysis membrane, and the diffusion of the substance in the tissue. This is also true for the so-called ‘reverse microdialysis,’ in which an exogenous substance is introduced into the extracellular space via the probe, as is the case in the present study. According to data in our laboratory, the estimated recovery level of cocaine in the interstitial fluids under the present experimental conditions was ∼10–12% of their true perfusion concentration. This view is supported by the concentrations (8 nM) obtained in nucleus accumbens when N-methyl-[3H]-cocaine was infused via microdialysis. Thus, it seems reasonable to conclude that the perfused (100 nM) cocaine concentration corresponds to an effective 10 nM concentration in rat nucleus accumbens, which is in line with the previous in vitro data (Ferraro et al, 2010).
The neurochemical results of this study taken together with our previous findings suggest that cocaine at low nanomolar concentrations could enhance not only D2-likeR agonist-mediated signaling at D2 autoreceptors in vitro (Ferraro et al, 2010), but also the inhibitory action of quinpirole at D2-likeRs regulating glutamate uptake and presynaptic D2-like heteroreceptors on glutamate terminals in vivo and/or at postjunctional D2-likeRs in the ventral striato-pallidal GABA neurons that inhibit glutamate release in vivo via increased synthesis and release of endocannabinoids that activate inhibitory CB1 receptors on glutamate terminals (Uchigashima et al, 2007). The neurochemical scenario for the allosteric actions of cocaine at the D2-likeR within the nucleus accumbens observed in microdialysis assays seems to also be associated with behavioral consequences as suggested by the results presented here. In line with previous results (Klimek and Maj, 1989; Maj et al, 1989), quinpirole in a dose-range of 0.5–1 mg/kg significantly increased basal locomotor activity in rats. This behavioral action of quinpirole is known to depend on its stimulation of accumbal postjunctional D2Rs as evidenced from local microinfusion studies in adult rats with lesions of ascending DA pathways (Breese et al, 1987; Archer et al, 2003). Pretreatment with cocaine at a low and behaviorally inactive dose (0.625 mg/kg) produced a significant enhancement of quinpirole-induced locomotor hyperactivity thereby supporting the neurochemical data obtained through microdialysis. The possibility that this effect may be due to cocaine-induced blockade of the DA transporter seems to be ruled out as GBR 12783 did not enhance quinpirole-induced hyperactivity. These observations are in agreement with the postulated direct allosteric enhancing actions of cocaine at postjunctional D2Rs and/or presynaptic D2 heteroreceptors or indirect allosteric actions via associated proteins and/or discrete lipids. The demonstration that selective 6-OHDA-induced destruction of the meso-limbic DA neurons blocks cocaine-induced locomotion (Kelly and Iversen, 1976) is compatible with this view as the enhancing allosteric action of cocaine may not develop without the presence of DA or a D2R agonist activating the orthosteric site of the D2R to a significant degree. Further experiments involving intra-accumbens infusions of quinpirole and cocaine, alone and in combination, are necessary to assess whether the observed effects on locomotor activity are nucleus accumbens specific.
These in vivo effects observed with cocaine may at least in part involve the ability of the drug to increase the efficacy of agonist-induced activation of D2-likeRs in view of the increased efficacy of DA-stimulated D2-likeR activation in striatal membranes in the presence of cocaine using the GTPγS binding assay. The results suggest that the effect produced by cocaine is independent of DA transporter blockade as there was no increase in the efficacy of DA-stimulated D2-likeR-Gi-protein coupling in the presence of 200 nM GBR 12783, a very potent and selective inhibitor of DA transporter (Bonnet and Costentin, 1986). In addition, the significant increase in the maximal response to DA-stimulated GTPγS binding to D2-likeRs by 100 nM cocaine remained in the presence of DA transporter blockade by GBR 12783.
On the contrary, the possibility that cocaine increases agonist binding to striatal D2-likeRs seems to be ruled out by the negative results obtained in competition experiments in striatal membranes performed with quinpirole and DA using the D2-likeR antagonist [3H]raclopride. It is worth noting that our findings with GTPγS are in line with a previous study demonstrating that in D2LR stably transfected CHO cells lacking the DA transporter, cocaine produced a small, but significant increase in DA-stimulated binding of GTPγS (Marcellino et al, 2010). Importantly, in that study the working cocaine concentration (150 μM) increased the affinity of D2R agonist binding sites.
In conclusion, to the best of our knowledge the results of this study demonstrate for the first time that cocaine at a concentration (100 nM) can enhance inhibitory effects of quinpirole on accumbal extracellular glutamate levels in the awake rat. In addition, cocaine at a dose (0.625 mg/kg) lacking effects on locomotion on its own, produces a significant enhancement of quinpirole- (1 mg/kg) induced hyperlocomotion. A cocaine-induced enhancement of G-protein coupling to D2-likeRs found in vitro in striatal membranes may at least in part underlie these in vivo neurochemical and behavioral effects. Together with previous in vitro findings (Ferraro et al, 2010; Genedani et al, 2010; Marcellino et al, 2010), these results suggest the existence of a direct allosteric enhancing action of cocaine at D2-likeRs and/or indirect actions via associated proteins and/or discrete lipids in vivo. Moreover, our findings of novel cocaine actions may have relevance for understanding the actions of cocaine upon accumbal DA and/or glutamate transmission participating in reward and relapse and thus its addictive properties.
References
Agnati LF, Leo G, Zanardi A, Genedani S, Rivera A, Fuxe K et al (2006). Volume transmission and wiring transmission from cellular to molecular networks: history and perspectives. Acta Physiol (Oxf) 187: 329–344.
Archer T, Palomo T, McArthur R, Fredriksson A (2003). Effects of acute administration of DA agonists on locomotor activity: MPTP versus neonatal intracerebroventricular 6-OHDA treatment. Neurotox Res 5: 95–110.
Baker DA, Shen H, Kalivas PW (2002). Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids 23: 161–162.
Bonnet JJ, Costentin J (1986). GBR 12783, a potent and selective inhibitor of dopamine uptake: biochemical studies in vivo and ex vivo. Eur J Pharmacol 121: 199–209.
Breese GR, Duncan GE, Napier TC, Bondy SC, Iorio LC, Mueller RA (1987). 6-hydroxydopamine treatments enhance behavioral responses to intracerebral microinjection of D1- and D2-dopamine agonists into nucleus accumbens and striatum without changing dopamine antagonist binding. J Pharmacol Exp Ther 240: 167–176.
Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P (2001). Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci 13: 1071–1077.
De Mei C, Ramos M, Iitaka C, Borrelli E (2009). Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol 9: 53–58.
Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C et al (2004). Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47 (Suppl 1): 227–241.
Di Chiara G, Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278.
Ferraro L, Antonelli T, O'Connor WT, Fuxe K, Soubrie' P, Tanganelli S (1998). The striatal neurotensin receptor modulates striatal and pallidal glutamate and GABA release: functional evidence for a pallidal glutamate-GABA interaction via the pallidal—subthalamic nucleus loop. J Neurosci 18: 6977–6989.
Ferraro L, Beggiato S, Marcellino D, Frankowska M, Filip M, Agnati LF et al (2010). Nanomolar concentrations of cocaine enhance D2-like agonist-induced inhibition of the K+-evoked [3H]-dopamine efflux from rat striatal synaptosomes: a novel action of cocaine. J Neural Transm 117: 593–597.
Filip M, Frankowska M, Zaniewska M, egaliñski E, Muller CE, Agnati L et al (2006). Involvement of adenosine A2A and dopamine receptors in the locomotor and sensitizing effects of cocaine. Brain Res 1077: 67–80.
Frankowska M, Nowak E, Filip M (2009). Effects of GABAB receptor agonists on cocaine hyperlocomotor and sensitizing effects in rats. Pharmacol Rep 61: 1042–1049.
Fuxe K, Dahlstrom A, Hoistad M, Marcellino D, Jansson A, Rivera A et al (2007). From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: Wiring and volume transmission. Brain Res Rev 55: 17–54.
Fuxe K, Dahlstrom A, Jonsson G, Marcellino D, Guescini M, Dam M et al (2009). The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog Neurobiol 90: 82–100.
Genedani S, Carone C, Guidolin D, Filaferro M, Marcellino D, Fuxe K et al (2010). Differential sensitivity of A2A and especially D2 receptor trafficking to cocaine compared with lipid rafts in cotransfected CHO cell lines. Novel actions of cocaine independent of the DA transporter. J Mol Neurosci 41: 347–357.
Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D (1999). Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2: 358–363.
Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H et al (2000). D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci 20: 8987–8995.
Kalivas P, Duffy P (1997). Dopamine regulation of extracellular glutamate in the nucleus accumbens. Brain Res 761: 173–177.
Kalivas PW, Volkow ND (2005). The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162: 1403–1413.
Kelly PH, Iversen SD (1976). Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol 40: 45–56.
Kerkerian L, Dusticier A, Nieoullon A (1987). Modulatory effect of dopamine on high-affinity glutamate uptake in the rat striatum. J Neurochem 48: 1301–1306.
Klimek V, Maj J (1989). Repeated administration of antidepressants enhances agonist affinity for mesolimbic D2-receptors. J Pharm Pharmacol 41: 555–558.
Koob GF, Bloom FE (1988). Cellular and molecular mechanisms of drug dependence. Science 242: 715–723.
Kreitzer AC, Malenka RC (2005). Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci 25: 10537–10545.
LaLumiere RT, Kalivas PW (2008). Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci 28: 3170–3177.
Lee FJ, Pei L, Liu F (2009). Disruption of the dopamine transporter-dopamine D2 receptor interaction in schizophrenia. Synapse 63: 710–712.
Maj J, Papp M, Skuza G, Bigajska K, Zazula M (1989). The influence of repeated treatment with imipramine, (+)- and (-)-oxaprotiline on behavioural effects of dopamine D-1 and D-2 agonists. J Neural Transm 76: 29–38.
Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I et al (2008). Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology 54: 815–823.
Marcellino D, Narvarro G, Sahlholm K, Nilsson j, Agnati L, Lluis C et al (2009). Cocaine Functions as a Possible Allosteric Agonist at Dopamine Receptors. SfN Abstract. Society for Neuroscience: San Diego, CA, USA (online).
Marcellino D, Navarro G, Sahlholm K, Nilsson J, Agnati LF, Canela EI et al (2010). Cocaine produces D2R-mediated conformational changes in the adenosine A(2A)R-dopamine D2R heteromer. Biochem Biophys Res Commun 394: 988–992.
Martire A, Tebano MT, Chiodi V, Ferreira SG, Cunha RA, Köfalvi A et al (2011). Pre-synaptic adenosine A2A receptors control cannabinoid CB1 receptor-mediated inhibition of striatal glutamatergic neurotransmission. J Neurochem 116: 273–280.
Nieoullon A, Kerkerian L, Dusticier N (1982). Inhibitory effects of dopamine on high affinity glutamate uptake from striatum. Life Sci 30: 1165–1172.
Paxinos G, Watson C (1986). The Rat Brain in Stereotaxic Coordinates. Academic Press: New York.
Pickel VM, Chan J, Kearn CS, Mackie K (2006). Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol 495: 299–313.
Rice ME, Cragg SJ (2008). Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev 58: 303–313.
Rinken A, Finnman UB, Fuxe K (1999). Pharmacological characterization of dopamine-stimulated [35S]-guanosine 5′(gamma-thiotriphosphate) ([35S]GTPgammaS) binding in rat striatal membranes. Biochem Pharmacol 57: 155–162.
Saulskaya NB, Mikhailova MO (2002). Feeding-induced decrease in extracellular glutamate level in the rat nucleus accumbens: dependence on glutamate uptake. Neuroscience 112: 791–801.
Saulskaya NB, Soloviova NA (2004). Tetrodotoxin-dependent glutamate release in the rat nucleus accumbens during concurrent presentation of appetitive and conditioned aversive stimuli. J Neurosci Methods 140: 15–21.
Schneider JS, Wade T, Lidsky TI (1998). Chronic neuroleptic treatment alters expression of glial glutamate transporter GLT-1 mRNA in the striatum. NeuroReport 9: 133–136.
Self DW (2010). Dopamine receptor subtypes in reward and relapse. In: Neve KA (ed). The Dopamine Receptors, 2nd edn. Humana Press: Totowa, NJ, USA, pp 479–525.
Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV (1999). Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav 64: 327–336.
Tanganelli S, Sandager Nielsen K, Ferraro L, Antonelli T, Kehr J, Franco R et al (2004). Striatal plasticity at the network level. Focus on adenosine A2A and D2 interactions in models of Parkinson's Disease. Parkinsonism Relat Disord 10: 273–280.
Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M (2007). Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci 27: 3663–3676.
Woodward JJ, Compton DM, Balster RL, Martin BR (1995). In vitro and in vivo effects of cocaine and selected local anesthetics on the dopamine transporter. Eur J Pharmacol 277: 7–13.
Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW (2002). Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther 300: 162–171.
Yin HH, Lovinger DM (2006). Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci USA 103: 8251–8256.
Zaniewska M, McCreary AC, Wydra K, Filip M (2010). Differential effects of serotonin (5-HT)2 receptor-targeting ligands on locomotor responses to nicotine-repeated treatment. Synapse 64: 511–519.
Acknowledgements
This work has been supported by grants from the Swedish Research Council (04x-715) and Hjärnfonden (Uppsala, Sweden) as well as statutory funds of the Institute of Pharmacology, Polish Academy of Sciences (Kraków, Poland).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ferraro, L., Frankowska, M., Marcellino, D. et al. A Novel Mechanism of Cocaine to Enhance Dopamine D2-Like Receptor Mediated Neurochemical and Behavioral Effects. An In Vivo and In Vitro Study. Neuropsychopharmacol 37, 1856–1866 (2012). https://doi.org/10.1038/npp.2012.33
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2012.33
Keywords
This article is cited by
-
Cocaine Modulates the Neuronal Endosomal System and Extracellular Vesicles in a Sex-Dependent Manner
Neurochemical Research (2022)
-
Acute Cocaine Enhances Dopamine D2R Recognition and Signaling and Counteracts D2R Internalization in Sigma1R-D2R Heteroreceptor Complexes
Molecular Neurobiology (2019)
-
In Vitro Effects of Cocaine on Tunneling Nanotube Formation and Extracellular Vesicle Release in Glioblastoma Cell Cultures
Journal of Molecular Neuroscience (2015)
-
On the role of adenosine (A)2A receptors in cocaine-induced reward: a pharmacological and neurochemical analysis in rats
Psychopharmacology (2015)
-
Chronic Δ9-Tetrahydrocannabinol Exposure Induces a Sensitization of Dopamine D2/3 Receptors in the Mesoaccumbens and Nigrostriatal Systems
Neuropsychopharmacology (2012)