Abstract
The ability to infer value from the reactions of other people is a common and essential ability with a poorly understood neurobiology. Commonly, social learning matches one's values and behavior to what is perceived as normal for one's social group. This is known as conformity. Conformity of value correlates with neural activity shared by cognitions that depend on optimum catecholamine levels, but catecholamine involvement in conformity has not been tested empirically. Methylphenidate (MPH) is an indirect dopamine and noradrenalin agonist, commonly used for the treatment of attention-deficit hyperactivity disorder for which it reduces undesirable behavior as evaluated by peers and authority figures, indicative of increased conformity. We hypothesized that MPH might increase conformity of value. In all, 38 healthy adult females received either a single oral 20 mg dose of MPH or placebo (PL). Each subject rated 153 faces for trustworthiness followed immediately by the face's mean rating from a group of peers. After 30 min and a 2-back continuous-performance working-memory task, subjects were unexpectedly asked to rate all the faces again. Both the groups tended to change their ratings towards the social norm. The MPH group exhibited twice the conformity effect of the PL group following moderate social conflict, but this did not occur following large conflicts. This suggests that MPH might enhance signals that would otherwise be too weak to evoke conformity. MPH did not affect 2-back performance. We provide a new working hypothesis of a neurocognitive mechanism by which MPH reduces socially disruptive behavior and provides novel evidence of catecholamine mediation of social learning.
Similar content being viewed by others
INTRODUCTION
From food to politics, our choices and behaviors are guided by the values we associate with the available options. The ability to infer the value of an option from opinions expressed by others is an essential skill. This skill enables us to learn quickly about options without the costs that accompany trial and error. It allows values to be taught intentionally and passed from one generation to the next. It even enables us to build representations of other people's desires so that we can cooperate effectively and enhance our own reputations. Remarkably little is known about how social learning occurs, despite its common occurrence across species (Galef and Laland, 2005). This study investigates the role of catecholamines in the social learning process. The results provide new insight into pharmacological treatments of social learning deficits and increase our understanding of the social impact of certain pharmacological events.
When social learning matches one's values and behavior to what is perceived as normal for one's social group, it is conformity. Conformity can be motivated by gains of reputation, gains of knowledge, or both (Cialdini and Goldstein, 2004; Deutch and Gerard, 1955). Either can lead to more positive and fewer negative future outcomes (Fehr and Fischbacher, 2004; Turner, 1991). Conformity of value can therefore involve learning about the value of an object or event, as well as the value of agreement with others (Campbell-Meiklejohn et al, 2010). Correspondingly, studies have consistently reported that cognitive components of conformity can be tracked in the activity of brain regions known to mediate reinforcement learning. These include responses to social agreement (Campbell-Meiklejohn et al, 2010), conformity-inducing conflict (Berns et al, 2010; Campbell-Meiklejohn et al, 2010; Klucharev et al, 2009), changes of reputation (Izuma et al, 2008; Zink et al, 2008), and socially induced changes of object value (Campbell-Meiklejohn et al, 2010; Mason et al, 2009; Zaki et al, 2011). In nonsocial domains, task performance related to the same fronto-striatal circuitry is known to be sensitive to levels of catecholamine activity (Berridge, 2007; Clatworthy et al, 2009; Cools et al, 2001; Del Campo et al, 2011; Robbins and Arnsten, 2009), but catecholamine mediation of conformity of value has not yet been established.
Methylphenidate (MPH) is an indirect catecholamine agonist that increases extracellular dopamine and noradrenalin levels in the brain (Berridge et al, 2006; Volkow et al, 2001) with consequences for learning and other cognitions. With respect to value, MPH can enhance phasic dopamine responses to external stimuli and associated interest in rewards and tasks (Volkow et al, 2002, 2004), modulate flexible adaptation of stimulus–reinforcement association (Clatworthy et al, 2009), and alter reinforcement-associated synaptic plasticity and behavior (Tye et al, 2010). In other domains, it can alter cognitive performance that is dependent on optimum catecholamine levels including working memory, vigilance, and response inhibition (Arnsten, 2011; Del Campo et al, 2011; Swanson et al, 2011). MPH could therefore affect conformity to the extent that it depends on any of these processes.
Clinically, MPH is a very common treatment of attention deficit hyperactivity disorder (ADHD) (Wilens, 2008). It is also increasingly abused as a nonprescribed ‘cognitive enhancer’ (Smith and Farah, 2011). For ADHD patients characterized by inappropriate social behavior as evaluated by authority figures and peers, MPH and other stimulants tend to improve social performance (Gadow et al, 1995; Hinshaw et al, 1989; Klein et al, 1997; Pelham et al, 1985; Spencer et al, 2005; Sprague and Sleater 1977; Whalen et al, 1989). This effect may occur, in part, from increased conformity.
As evident from an infant observing a mother's reaction to a stranger (Feinman et al, 1992), a particularly common and important socially learned value is trust. Trust is the value of a social interaction based on the perceived likelihood that the other party will act in one's best interests. Trustworthiness judgments of faces are good approximations of the overall valence (positive/negative) of face stimuli and reflective of approach/avoidance decisions in the absence of clear emotional cues of the other person's intentions (Todorov, 2008).
Given the overlap of neural activities supporting conformity and nonsocial catecholamine-mediated cognition, we anticipated that MPH might affect conformity of value. Because MPH enhances reinforcement saliency in healthy adults and makes behavior of patients more acceptable to one's peers and authority figures, we anticipated that the effect would be to increase conformity. We adapted a task shown to be sensitive susceptibility to social influence (Klucharev et al, 2009, 2011; Zaki et al, 2011) to test for the effect of MPH on conformity of trustworthiness ratings of faces.
MATERIALS AND METHODS
Recruitment and Screening
The ethics committee of Central Jutland Region, Denmark, reviewed the methods and approved this study. Subjects were recruited with posters and newspaper advertisements. Prospective subjects were given a physical examination by a physician and screened according to certain criteria before being accepted onto the study. To be accepted onto the study, subjects had to be nonsmokers, female, between the ages of 18 and 35, free of current DSM-IV illness, free of history of major depression, free of psychotropic drugs (except for oral contraception) for 3 months, free of head injury or stroke, and free of any illness or medication associated with adverse interactions with an acute dose of MPH. Only women were used in this study because the faces of the experiment's task were exclusively of women and we wished to avoid confounds of cross-gender effects on trustworthiness ratings.
Subjects: Demographics, State, and Trait Measures
Thirty-eight healthy women were matched for age (M 23, SD 2.7), years of education (M 13.8, SD 1.8), performance intelligence (WAIS-III Matrix Reasoning, scaled M 11.53, SD 1.7), and verbal intelligence (WAIS-III Vocabulary, scaled M 12.7, SD 1.7) (Wechsler, 2005), and randomly assigned to one of the two drug groups by an independent third party. Before the test day, trait measures of anxiety (Spielberger, 1970), social anxiety (Liebowitz, 1987), self-monitoring (Snyder and Gangestad, 1986), and mood (Watson et al, 1988) were recorded for each subject. State measures of anxiety (Spielberger, 1970), mood (Watson et al, 1988), nausea, and headache were recorded immediately before receiving the drug or placebo (PL) and again just prior to the testing.
Procedure
In a double-blind procedure, one group received a single 20 mg oral dose of MPH, whereas the other received a PL. Subjects were tested within the first 12 days of their menstrual cycle and were asked to refrain from caffeine 24 h prior to the study. One hour after receiving the drug, subjects performed the first session of the conformity task. This took about 12 min. Next, subjects performed a 2-back continuous-performance working-memory task (Owen et al, 2005) and an unrelated gambling task (Campbell-Meiklejohn et al, 2011). At 30 min after the initial ratings, subjects were surprised with the second session of the conformity task. Subjects did not know about the second session until this point. The second session took approximately 8 min. Both the cognitive tasks were presented to subjects on a computer using Presentation v. 14 (Neurobehavioral Systems).
Conformity Task
The task (Figure 1) was similar to that described previously (Klucharev et al, 2009, 2011; Zaki et al, 2011). Subjects rated 153 female faces with moderate smile and attractiveness on a scale from 1 (not at all) to 8 (very), for how ‘trustworthy’ they believed the owner of the face to be. The faces were presented in a random order. After rating each face, subjects were told the average rating of that face by other subjects performing the task at other European universities. This group rating is the ‘social norm.’ The mean initial rating of the faces by subjects was 4.8±0.4 (SD). Subtraction of subject ratings from the social norm resulted in our independent variable of ‘social conflict’ for each face. To enable comparisons with neuroimaging studies that used similar tasks (Klucharev et al, 2009, 2011; Zaki et al, 2011), we used the same five social conflict conditions as those studies. Subjects could be in ‘no conflict’ (NC) (within 1, including zero), moderate social conflict (−2, +2), or high social conflict (−3, +3) with the social norm. Social conflict was negative if the social norm had a lower rating than the subject, and positive if the social norm was higher. Subjects were informed that the visual feedback for −1, 0, and +1 social conflict would be identical.
Conformity Task. Subjects rated 153 faces, one by one, for their expected trustworthiness on a scale from 1 to 8, indicated by pressing the corresponding number on a keyboard. The choice was highlighted by a green vertical rectangle. After rating each face, subjects learned the ‘social norm’ rating of that face, which subjects were told was the average rating from four identical studies in European universities. The social norm rating was highlighted by a horizontal blue rectangle so that overlap with the subject's response could be observed. Social conflict with the norm could be ‘no conflict’, moderate (±2), or high (±3). Subjects were told that low conflict (±1) would be displayed as ‘no conflict’. Unexpectedly, subjects rated the faces again after 30 min, in a random order, without social feedback. Display was presented to subjects in color.
Unbeknownst to subjects, social conflict was not real. It was anchored to the subject's rating to ensure that enough events occurred in each social conflict condition to make a reliable estimate of conformity. This provided approximately 22 faces with a social conflict of −3, 28 with −2, 52 with NC, 30 with +2, and 20 with +3. Thirty-seven subjects expressed no doubts about the study's story authenticity, and one reported only mild doubt when debriefed at the end of the experiment.
The surprise second session, 30 min later, was a rerating of all the faces for trustworthiness on the same scale, in random order, without social norm feedback.
Dependent Measures of Conformity
We recorded the change of trustworthiness rating from before to after learning the social norm rating of each face (between sessions).
Copin is the mean change of face rating. We calculated Copin for each of the five social conflict conditions (−3, −2, NC, +2, and +3). Copin was positive if the subject's second rating was higher, on average, than their initial rating and negative if the second rating was lower.
Ctwrd is the mean change of face rating towards the social norm. Ctwrd was positive if the subject's rating changed, on average, towards the social norm and negative if it changed away. We calculated Ctwrd values for all social conflict levels (−3, −2, +2, and +3) and conflict conditions collapsed by magnitude (±2, ±3).
Reaction time and probability of conformity were also recorded.
2-Back
Subjects performed the 2-back task at the time of screening (baseline) and between the sessions of the conformity task. The 2-back is a continuous performance measure of sustained attention and working memory. Subjects are asked to press a button when the displayed letter (one out of five possible) matches the letter displayed 2 letters earlier in a continuous sequence. These letters were targets. Each letter was displayed for 2 s on a computer display in a series of 152 trials. The letters were presented in a random order. Correct indication is a ‘hit,’ missing an indication is a ‘miss’, and incorrect indication is a ‘false alarm.’ Changes of hits, misses, and false alarms before and after drug or PL treatment were the dependent measures of this study.
Statistical Analysis
All tests were performed with SPSS 19.0 (IBM). Normality of the dependent variables was determined by a statistical threshold of kurtosis and skewness statistics (Z<1.96). Mann–Whitney U-tests were used in place of independent t-tests when normality assumptions were violated. Where assumptions of sphericity were deemed violated by Mauchley's test in repeated-measures analysis of variance (ANOVA), degrees of freedom were corrected by Greenhouse–Geisser estimates.
Demographic, states, and traits
Independent sample t-tests were used to test for any between-group differences on demographic, state, or trait measures.
Conformity
To test if Copin was related to by social conflict, drug treatment, or an interaction between the two, we used a mixed design ANOVA. The within-subject factor was social conflict (five levels: −3, −2, NC, +2, and +3) and the between-subject factor was drug group (MPH vs PL). In case of ceiling effects, we performed a similar analysis for Copin in the range of moderate conflict (only three within-subject levels: −2, NC, and +2).
To compare if the conformity differed between positive and negative social conflict, we used paired t-tests of Ctwrd following +2 vs −2 and +3 vs −3. Because no differences were observed (Ps>0.33), we used the more reliable (more trials) Ctwrd values of ±2 and ±3 for subsequent analysis. A single outlier with a very large Ctwrd value following moderate (±2) social conflict was changed to the ±2 Ctwrd value (plus 0.01) of the participant with the next highest value to ensure homogeneity of variance between groups, as recommended (Field, 2009). A mixed design ANOVA was performed on Ctwrd with social conflict (±2, ±3) as a within-subject factor and drug group as a between-subject factor. Independent sample t-tests were then done to further investigate for differences of Ctwrd between drug groups following moderate (⩽±2), and separately, following high (±3) social conflict.
To test if conformity was more frequent following high (vs moderate) social conflict, a repeated-measures ANOVA was used to compare the mean probability of a change towards the social norm between the two conditions across all subjects. To ensure that effects did not emerge from group differences of original ratings or tendencies to change opinions, independent sample t-tests were used to compare mean initial trustworthiness ratings and Copin on NC trials between drug groups. Reaction times were tested between groups by independent sample t-tests in each social conflict condition for both sessions.
Effects on fatigue
To check that group differences of conformity were not due to prevention of fatigue by MPH, correlation coefficients were calculated for trial number × reaction time and for trial number × Ctwrd following moderate social conflict for each subject. The resulting correlation coefficients were tested for differences between drug groups by independent sample t-tests.
2-back
The effect of drug on 2-back performance was tested as (a) a main effect of drug group on each dependent measure during post-treatment performance by independent sample t-test; and (b) the interaction of the effects of drug and session on each dependent measure from a mixed model repeated-measures ANOVA with session as a within-subject factor and drug group as a between-subject factor. Repeated-measures ANOVAs were used to confirm equal number of targets in both sessions, in both groups. Independent sample t-tests confirmed equal baseline performance in each group.
RESULTS
Conformity behavioral measures are summarized in Table 1. N-back behavioral measures are summarized in Table 2. Trait and state measures are summarized in Supplementary Tables S1 and S2 (see Supplementary Information).
Demographic State and Trait Measures
MPH subjects increased positive mood more than PL subjects after treatment (t(36)=−3.2, P<0.01), but positive mood did not significantly differ between-groups at the time of testing and the change in mood did not correlate with the measures of conformity (Ps>0.2). No other demographic, trait, or state measure differed between drug groups or correlated with conformity.
Conformity Effects
We observed a main effect of social conflict (five levels: −3, −2, NC, +2, and +3) on Copin (F(4, 144)=87.95, P<0.001). This confirmed that a relationship between social conflict and changes of opinion on the conformity task.
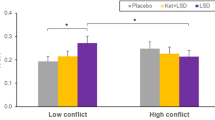
No overall interaction was observed between the effects of social conflict and drug group on Copin across the full range of conflicts (five levels: −3, −2, NC, +2, and +3) (P>0.18). Within the range of moderate social conflict (three levels: −2, NC, and +2), however, a significant interaction between the effects of social conflict and drug group on Copin was observed (F(1.58, 56.9)=3.43, P<0.05) (Figure 2a). This range still contained a strong main effect of social conflict on Copin (F(1.58, 56.9)=35, P<0.001). This established an effect of drug on conformity within the range of moderate social conflict.
Methylphenidate Effect on Conformity. Subjects treated with methylphenidate conformed more following moderate social conflict. (a) Change of value (Copin) for moderate social conflict magnitudes of −2, NC, and 2. Error bars reflect one within-subject SE. (b) Change of value towards social norm (Ctwrd) for moderate (±2) and high (±3) social conflict conditions. Error bars reflect one between-subject SE.
We observed a main effect of social conflict (moderate (±2) vs high (±3)) on Ctwrd (F(1, 36)=62.35, P<0.001). Subjects conformed more if the conflict was high. In the same analysis, we observed an interaction between the effects of social conflict and drug group on Ctwrd (F(1, 36)=4.67), P<0.039). Independent sample t-tests established that this interaction was due to MPH having no effect on Ctwrd after high conflict (P>0.8) but evoking twice the Ctwrd of PL after moderate conflict (t(36)=2.4, P<0.022) (Figure 2b).
Initial trustworthiness ratings and experienced social conflict were similar between drug groups (Ps>0.38). In the NC condition, Copin did not differ between drug groups, which indicated that there was no group difference of a general tendency to change opinion between sessions. We did not observe differences of reaction time between drug groups in any social conflict condition (Ps>0.16). High (vs moderate) conflict generated a greater mean probability of conformity than moderate conflict across all subjects (F(1, 37)=18.6, P<0.001).
Fatigue
Correlations between trial number × reaction time and trial number × Ctwrd following moderate conflict did not differ between MPH and PL groups (Ps>0.25). Conformity results were therefore unlikely to result from MPH effects on fatigue.
2-Back
Drug groups did not differ on any 2-back performance measure before or after treatment (Ps>0.25) (Table 2). Subjects generally improved on each dependent measure of the 2-back task between pre and post treatment (hits: F(1, 36)=14.9, P<0.001; misses F(1, 36)=12.6, P<0.001; and false alarms: F(1, 36)=5.4, P<0.03), but groups showed similar improvements (Ps>0.25). Targets were presented equally often in both groups (P>0.5) and sessions (P>0.2). The effect of MPH on conformity was therefore unlikely to result from a general effect on working memory or sustained attention.
DISCUSSION
The conformity task elicited conformity in both groups as predicted by previous studies using a similar task (Klucharev et al, 2009, 2011; Zaki et al, 2011). The tendency to increase magnitude and probability of conformity reflects the findings of early conformity research (Fisher and Lubin, 1958; Goldberg, 1954; Hovland and Pritzker, 1957; Zimbardo, 1960). Subjects who received MPH exhibited twice the conformity of subjects receiving a PL after moderate social conflict without observed effects on working memory, sustained attention, or fatigue. This provides initial pharmacological evidence that catecholamine systems could underlie conformity to social norms found in previous neuroimaging studies (Campbell-Meiklejohn et al, 2010; Klucharev et al, 2009). We found no evidence that the conformity results reflected MPH effects on sustained attention, working memory, or fatigue.
A mechanism of MPH effects on social influence is suggested by prior research. A low oral dose of MPH has been shown to increase the levels of extracellular dopamine in the striatum in response to appetitive stimuli, indicative of stimulus-driven phasic dopamine release (Volkow et al, 2001, 2002, 2004; Wightman and Robinson, 2002). This increase is accompanied by increases of stimuli desirability, interest, and motivation during cognitive tasks (Volkow et al, 2002, 2004). Volkow et al (2005) have proposed that MPH amplifies dopaminergic responses to appetitive stimuli that would otherwise be of insufficient strength to establish enough salience to interest the subject. For the current results, we suggest that MPH may amplify dopamine responses to cues for moderate gains of conformity-based rewards that would otherwise lack the necessary strength or duration to reliably change a face rating. In contrast, relatively larger conflicts may generate signals of sufficient strength to reliably induce conformity without enhancement by MPH. Such a hypothesis assumes that the incentive salience of conformity increases with the size of social conflict and that a threshold of salience needs to be met to induce conformity. Our finding that conformity was also more frequent when conflict was high, as compared with moderate, supports this case. Further support for the incentive of conformity is gained from neuroimaging findings that agreement with others and gains of reputation evoke similar reward activity in the striatum to that of nonsocial rewards (Campbell-Meiklejohn et al, 2010; Izuma et al, 2008; Zink et al, 2008). The hypothesis also assumes that the magnitude of a cued incentive is carried by phasic dopamine signals, which has been shown previously (Tobler et al, 2005). In future work, modulation of phasic dopamine responses by factors already known to increase the incentive of conformity, such as group size and consensus among the group (Asch, 1951), would further support this interpretation.
Subjects did not differ on a 2-back task reflecting working memory and sustained attention performance. This reduces the likelihood that group differences of conformity arose from improvement on these measures. Null effects of MPH on the 2-back performance have been found previously (Mattay et al, 2000). In contrast, previous studies (Elliott et al, 1997; Mehta et al, 2000) have found that MPH can affect spatial working memory in healthy volunteers. One possible explanation for the discrepancy with our results is that the two tasks have different cognitive requirements (Smith and Farah, 2011). Another potential explanation is experience. Elliott et al (1997) found that MPH could improve spatial working-memory performance on the first exposure to the task but impair it on the second. Our subjects performed the task before and after drug administration so that we could account for baseline cognitive ability. It is possible that one might find a different result if MPH-treated subjects only encountered the 2-back once, but such a study would not account for baseline performance.
While the suggested mechanism centres on the activity of the basal ganglia and dopamine, effects of MPH on prefrontal cortex and noradrenalin should not be discounted (Arnsten, 2011). Microdialysis studies in rats have shown that MPH has pronounced effects on catecholamine levels of the prefrontal cortex (Berridge et al, 2006). In humans, MPH has been shown to bind to noradrenalin transporters (Hannestad et al, 2010), but this is not yet demonstrated in the frontal cortex because of limitations of positron emission tomography. Prefrontal cognitions affected by MPH include working memory (Elliott et al, 1997; Mehta et al, 2000) and response inhibition (Nandam et al, 2011). Studies also show that noradrenalin may mediate important functions of working memory, attentional set shifting, and response inhibition (Arnsten, 2011). Such cognitions could theoretically support conformity task performance but the precise contribution is not clear in the absence of MPH effects on the 2-back task and use of a wider range of control tasks. Future studies can build on this study work by comparing the effects of MPH with the effects of atomoxetine (a prefrontal specific catecholamine agonist) and observe interactions with specific dopamine and noradrenaline antagonists to isolate the neural structures and specific neurotransmitters involved. Given the distribution of cortical and striatal systems involved in social conformity (Berns et al, 2010; Campbell-Meiklejohn et al, 2010; Klucharev et al, 2009), we expect that, as for nonsocial learning (Kehagia et al, 2010), conformity involves a network of brain regions, multiple cognitions, and interactions between multiple neurotransmitter systems (Boureau and Dayan, 2011; Cools et al, 2011).
Although we observe a main effect across all subjects, MPH mediation of social learning may be dose-dependent and vary with individual differences of baseline tendency and baseline catecholamine activity (Clatworthy et al, 2009; Del Campo et al, 2011; Volkow et al, 2005). Effects for healthy adults may not be the same as for individuals with catecholamine deficits. Moreover, as this study demonstrates, stimulants may improve performance on some tasks but impair or have no effect on others, consistent with data suggesting inverted U-shaped relationships between levels of catecholamines and performance can vary between tasks (Arnsten, 2009; Clatworthy et al, 2009; Cools et al, 2001; Robbins and Arnsten, 2009; Yerkes and Dodson, 1908). Future work should explore the effects of different doses of MPH, baseline conformity tendency, and baseline catecholamine activity on conformity behavior.
One might argue that the effects we observe may not be ‘social’ because subjects performed the task on a computer. Performing a computer-based task will likely have different nuances to a true social interaction, and field studies are needed to determine if results deriving from this laboratory experiment extend to real world social interactions. All subjects, however, did report belief in the background story of the experiment, which includes a belief in the social nature of the task. Moreover, changes of value towards feedback ratings tend to be much higher in social versions of this task compared with those when subjects are told a computer-provided feedback, as demonstrated by Klucharev et al. (2009). The effects reported here are well within the range of the social version.
Given the length of time between ratings, the large number of faces, the rapid pace, the distracter tasks, the randomization of face order between sessions, and the fact that subjects did not expect to rerate the faces, it was very unlikely that subjects explicitly remembered their original ratings or associated social conflict at the time of the second rating. Given that changes of value are detectable in reinforcement circuitry a second after the social conflict (Campbell-Meiklejohn et al, 2010), we believe that changes of value proably occur during the first session and without a necessity to remember the conflict at the second session. Still, we do not completely rule out that a few faces and ratings could have been recognized at the times of the second rating, and explicit processes and recognition memory may factor slightly into the results.
The etiology of social deficits in disorders such as ADHD and reasons for therapeutic effectiveness of MPH to alleviate these deficits is not established by these findings in healthy adults. However, it is plausible that stimulant-induced increases of conformity could contribute to MPH effects on social behavior. An MPH-induced increase in the incentive of conformity, for example, would be consistent with theories and findings that patients with ADHD have reinforcement learning deficits that can be alleviated by stimulant therapy (Frank et al, 2007; Haenlein and Caul, 1987; Luman et al, 2010; Volkow et al, 2005; Wilkison et al, 1995), enhanced responses to raised incentives (Andreou et al, 2007; Kohls et al, 2009; Luman et al, 2005), deficient psychophysical responding to affective stimuli that can be restored by stimulant medication (Conzelmann et al, 2011; Groen et al, 2009), and reduced hemodynamic responses to reward anticipation in the striatum (Plichta et al, 2009; Stark et al, 2011; Strohle et al, 2008). We therefore propose a new working hypothesis that MPH may improve social behavior and acceptance by peers in ADHD patients, in part, by increasing catecholamine-mediated conformity. The specificity of MPH effects on conformity following moderate conflict may translate to increases of patient learning from subtle social cues that would otherwise have little effect on values and behavior. When values become similar to values of the group, resulting behavior is more likely to be favorably viewed by group members and authority figures. A better understanding of reward deficits of ADHD (Luman et al, 2010) may informs our understanding of associated social deficits and their treatment.
Finally although we are beginning to shed light on ‘how’ subjects change our values, future studies still need to establish ‘why’ subjects conform more on MPH. Subjects may have altered their opinion because the social norm is considered a better indicator of value than their own deduction. Alternatively, subjects may have believed that holding opinions similar to the social norm will bring more associated rewards and less punishments from others in society (Cialdini and Goldstein, 2004; Fehr and Fischbacher, 2004). Reasons for conformity will also vary from person to person. It is difficult to distinguish reasons for conformity in behavior or neuroimaging of the reward system because different incentives can elicit similar responses. Creative experimental techniques are required to empirically tease the motivations apart.
Summary
We have presented novel evidence of catecholamine mediation in social learning. This is a critical step toward understanding the neurobiology of this essential social cognition. The results highlight a potential overlap in pharmacology of nonsocial and social learning worthy of future study in the lab and real world situations. Like the incentive value of appetitive stimuli, the incentive value of conformity may be enhanced by stimulant medication. The findings also provide a new working hypothesis of a neurocognitive mechanism by which MPH might help to reduce disruptive behavior as judged by peers and authority figures, through enhancement of conformity-related cognition.
References
Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A et al (2007). Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychol Med 37: 1703–1715.
Arnsten AF (2009). Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs 23 (Suppl 1): 33–41.
Arnsten AFT (2011). Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry 69: e89–e99.
Asch S (1951). Effects of group pressure upon the modification and distortion of judgement. In: Guetzkow H (ed). Groups, Leadership and Men. Carnegie Press: Pittsburgh.
Berns G, Capra C, Moore S, Noussair C (2010). Neural mechanisms of the influence of popularity on adolescent ratings of music. Neuroimage 49: 2687–2696.
Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B et al (2006). Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60: 1111–1120.
Berridge K (2007). The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 191: 391–431.
Boureau YL, Dayan P (2011). Opponency revisited: competition and cooperation between dopamine and serotonin. Neuropsychopharmacology 36: 74–97.
Campbell-Meiklejohn DK, Bach DR, Roepstorff A, Dolan RJ, Frith CD (2010). How the opinion of others affects our valuation of objects. Curr Biol 20: 1165–1170.
Campbell-Meiklejohn DK, Wakeley J, Herbert V, Cook J, Scollo P, Ray MK et al (2011). Serotonin and dopamine play complementary roles in gambling to recover losses. Neuropsychopharmacology 36: 402–410.
Cialdini R, Goldstein N (2004). Social influence: compliance and conformity. Annu Rev Psychol 55: 591–621.
Clatworthy PL, Lewis SJG, Brichard L, Hong YT, Izquierdo D, Clark L et al (2009). Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci 29: 4690–4696.
Conzelmann A, Woidich E, Mucha RF, Weyers P, Jacob CP, Lesch K-P et al (2011). Methylphenidate normalizes emotional processing in adult patients with attention-deficit/hyperactivity disorder: preliminary findings. Brain Res 1381: 159–166.
Cools R, Barker R, Sahakian B, Robbins T (2001). Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex 11: 1136–1143.
Cools R, Nakamura K, Daw ND (2011). Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology 36: 98–113.
Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW (2011). The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry 69: e145–e157.
Deutch M, Gerard HB (1955). A study of normative and informational social influences upon individual judgement. J Abnorm Psychol 51: 629–636.
Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW (1997). Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology (Berl) 131: 196–206.
Fehr E, Fischbacher U (2004). Third-party punishment and social norms. Evol Hum Behav 25: 63–87.
Feinman S, Roberts D, Hsieh K, Sawyer D, Swanson D (1992). A critical review of social referencing in infancy. In: Feinman S (ed). Social Referencing and the Social Construction of Reality in Infancy. Plenum Press: New York.
Field A (2009). Discovering Statistics Using SPSS. SAGE Publications Ltd: London.
Fisher S, Lubin A (1958). Distance as a determinant of influence in a two-person serial interaction situation. J Abnorm Psychol 56: 230–238.
Frank MJ, Santamaria A, O’Reilly RC, Willcutt E (2007). Testing computational models of dopamine and noradrenaline dysfunction in attention deficit/hyperactivity disorder. Neuropsychopharmacology 32: 1583–1599.
Gadow KD, Sverd J, Sprafkin J, Nolan EE, Ezor SN (1995). Efficacy of methylphenidate for attention-deficit hyperactivity disorder in children with tic disorder. Arch Gen Psychiatry 52: 444–455.
Galef B, Laland K (2005). Social learning in animals: empirical studies and theoretical models. Bioscience 55: 489–499.
Goldberg SC (1954). Three situational determinants of conformity to social norms. J Abnorm Psychol 49: 325–329.
Groen Y, Mulder LJM, Wijers AA, Minderaa RB, Althaus M (2009). Methylphenidate improves diminished error and feedback sensitivity in ADHD: an evoked heart rate analysis. Biol Psychol 82: 45–53.
Haenlein M, Caul WF (1987). Attention deficit disorder with hyperactivity: a specific hypothesis of reward dysfunction. J Am Acad Child Adolesc Psychiatry 26: 356–362.
Hannestad J, Gallezot JD, Planeta-Wilson B, Lin SF, Williams WA, van Dyck CH et al (2010). Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry 68: 854–860.
Hinshaw SP, Henker B, Whalen CK, Erhardt D, Dunnington RE (1989). Aggressive, prosocial, and nonsocial behavior in hyperactive boys: dose effects of methylphenidate in naturalistic settings. J Consult Clin Psychol 57: 636–643.
Hovland CI, Pritzker HA (1957). Extent of opinion change as a function of amount of change advocated. J Abnorm Psychol 54: 257–261.
Izuma K, Saito D, Sadato N (2008). Processing of social and monetary rewards in the human striatum. Neuron 58: 284–294.
Kehagia AA, Murray GK, Robbins TW (2010). Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol 20: 199–204.
Klein RG, Abikoff H, Klass E, Ganeles D, Seese LM, Pollack S (1997). Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry 54: 1073–1080.
Klucharev V, Hytonen K, Rijpkema M, Smidts A, Fernandez G (2009). Reinforcement learning signal predicts social conformity. Neuron 61: 140–151.
Klucharev V, Munneke MA, Smidts A, Fernandez G (2011). Downregulation of the posterior medial frontal cortex prevents social conformity. J Neurosci 31: 11934–11940.
Kohls G, Herpertz-Dahlmann B, Konrad K (2009). Hyperresponsiveness to social rewards in children and adolescents with attention-deficit/hyperactivity disorder (ADHD). Behav Brain Funct (BBF) 5: 20.
Liebowitz MR (1987). Social phobia. Mod Probl Pharmacopsychiatry 22: 141–173.
Luman M, Oosterlaan J, Sergeant JA (2005). The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev 25: 183–213.
Luman M, Tripp G, Scheres A (2010). Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci Biobehav Rev 34: 744–754.
Mason M, Dyer R, Norton M (2009). Neural mechanisms of social influence. Organ Behav Hum Decis Process 110: 152–159.
Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R et al (2000). Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage 12: 268–275.
Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW (2000). Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci 20: RC65.
Nandam LS, Hester R, Wagner J, Cummins TDR, Garner K, Dean AJ et al (2011). Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol Psychiatry 69: 902–904.
Owen AM, McMillan KM, Laird AR, Bullmore E (2005). N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25: 46–59.
Pelham WE, Bender ME, Caddell J, Booth S, Moorer SH (1985). Methylphenidate and children with attention deficit disorder. Dose effects on classroom academic and social behavior. Arch Gen Psychiatry 42: 948–952.
Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C et al (2009). Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 65: 7–14.
Robbins TW, Arnsten AF (2009). The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci 32: 267–287.
Smith ME, Farah MJ (2011). Are prescription stimulants ‘smart pills’? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychol Bull 137: 717–741.
Snyder M, Gangestad S (1986). On the nature of self-monitoring: matters of assessment, matters of validity. J Pers Soc Psychol 51: 125–139.
Spencer T, Biederman J, Wilens T, Doyle R, Surman C, Prince J et al (2005). A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 57: 456–463.
Spielberger CD (1970). STAI Manual. Consulting Psychologist Press.
Sprague RL, Sleator EK (1977). Methylphenidate in hyperkinetic children: Differences in dose effects on learning and social behavior. Science 198: 1274–1276.
Stark R, Bauer E, Merz CJ, Zimmermann M, Reuter M, Plichta MM et al (2011). ADHD related behaviors are associated with brain activation in the reward system. Neuropsychologia 49: 426–434.
Strohle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M et al (2008). Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage 39: 966–972.
Swanson J, Baler RD, Volkow ND (2011). Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology 36: 207–226.
Tobler PN, Fiorillo CD, Schultz W (2005). Adaptive coding of reward value by dopamine neurons. Science (New York, NY) 307: 1642–1645.
Todorov A (2008). Evaluating faces on trustworthiness: an extension of systems for recognition of emotions signaling approach/avoidance behaviors. Ann N Y Acad Sci 1124: 208–224.
Turner JC (1991). Social Influence. Open University Press: Buckingham.
Tye KM, Tye LD, Cone JJ, Hekkelman EF, Janak PH, Bonci A (2010). Methylphenidate facilitates learning-induced amygdala plasticity. Nat Neurosci 13: 475–481.
Volkow N, Wang G, Fowler J, Ding Y (2005). Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry 57: 1410–1415.
Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L et al (2001). Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 21: RC121.
Volkow ND, Wang G-J, Fowler JS, Logan J, Jayne M, Franceschi D et al (2002). Nonhedonic food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 44: 175–180.
Volkow ND, Wang G-J, Fowler JS, Telang F, Maynard L, Logan J et al (2004). Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am J Psychiatry 161: 1173–1180.
Watson D, Clark L, Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54: 1063–1070.
Wechsler D (2005). Weschler Adult Intelligence Scale, Danish Version, 3rd edn. Pearson Assessment.
Whalen CK, Henker B, Buhrmester D, Hinshaw SP, Huber A, Laski K (1989). Does stimulant medication improve the peer status of hyperactive children? J Consult Clin Psychol 57: 545–549.
Wightman RM, Robinson DL (2002). Transient changes in mesolimbic dopamine and their association with ‘reward’. J Neurochem 82: 721–735.
Wilens TE (2008). Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 28 (Suppl 2): S46–S53.
Wilkison PC, Kircher JC, McMahon WM (1995). Effects of methylphenidate on reward strength in boys with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 34: 897–901.
Yerkes R, Dodson J (1908). The relation of stimulus strength to rapidity of habit formation. J Comp Neurol Psychol 18: 459–482.
Zaki J, Schirmer J, Mitchell JP (2011). Social influence modulates the neural computation of value. Psychol Sci 22: 894–900.
Zimbardo PG (1960). Involvement and communication discrepancy as determinants of opinion conformity. J Abnorm Psychol 60: 86–94.
Zink C, Tong Y, Chen Q, Bassett D, Stein J, Meyer-Lindenberg A (2008). Know your place: neural processing of social hierarchy in humans. Neuron 58: 273–283.
Acknowledgements
The project was funded by the Danish Research Foundation and the Danish Council for Independent Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Campbell-Meiklejohn, D., Simonsen, A., Jensen, M. et al. Modulation of Social Influence by Methylphenidate. Neuropsychopharmacol 37, 1517–1525 (2012). https://doi.org/10.1038/npp.2011.337
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.337
Keywords
This article is cited by
-
Effect of other visible reviews’ votes and personality on review helpfulness evaluation: an event-related potentials study
Electronic Commerce Research (2022)
-
LSD-induced increases in social adaptation to opinions similar to one’s own are associated with stimulation of serotonin receptors
Scientific Reports (2020)
-
Socially Learned Attitude Change is not reduced in Medicated Patients with Schizophrenia
Scientific Reports (2019)
-
Catecholaminergic modulation of trust decisions
Psychopharmacology (2019)
-
Nature vs. nurture in human sociality: multi-level genomic analyses of social conformity
Journal of Human Genetics (2018)