Abstract
Although worldwide millions of people work prolonged hours, at adverse circadian phases, evidence suggests that cognitive function is impaired under these conditions with important societal consequences. In a double-blind placebo-controlled laboratory-based study, we investigated the effect of the wakefulness-promoting drug modafinil as a countermeasure against such neurobehavioral impairments induced by both prolonged wakefulness and circadian misalignment. Neurobehavioral performance, alertness, and sleep were studied in young healthy participants (N=18) who underwent a 25-day forced desynchrony protocol in which the period of the sleep-wakefulness cycle was scheduled to be 42.85 h (duration of each wakefulness episode: 28.57 h; sleep/rest episode: 14.28 h). Each waking day, participants were treated with either 400 mg modafinil, divided into three doses, or placebo, according to a randomized, parallel-group design. Treatment with modafinil significantly attenuated the performance decrements seen for several parameters including cognitive-psychomotor speed, visual attention and reaction times both with progressive hours awake and when working at adverse circadian phases. Subjective alertness and sleep parameters were similar between treatment groups, but modafinil-treated participants had fewer bouts of inadvertent sleep during scheduled waking. Modafinil reduced the neurobehavioral impairment associated with work, both during prolonged wakefulness and at adverse circadian phases, without adversely affecting subjective alertness or subsequent sleep. These features suggest that modafinil might be a particularly relevant countermeasure against the deleterious effects of prolonged work hours, shift work, and transmeridian travel.
Similar content being viewed by others
INTRODUCTION
Performance deficits and deterioration of mood occur when human beings are subjected to sleep loss and when they attempt to work at adverse circadian phases (Boivin et al, 1997; Dijk et al, 1992; Hull et al, 2003; Santhi et al, 2008; Wyatt et al, 1999). These cognitive impairments include slowed reaction times, increased error rates, reduced vigilance, memory decrements, poor motivation, increased variability in performance, as well as reduced subjective alertness, and subjective well-being. Certain individuals may be more susceptible to such impairments and a possible genetic basis for such susceptibility has been described (Bodenmann et al, 2009; Czeisler, 2009; Viola et al, 2007). Accumulating evidence suggests that the degradation of performance associated with sleep deprivation and working at an adverse circadian phase has important social implications including, but not limited to, compromised public safety, diminished health and well-being, and lower productivity of the affected population (Åkerstedt et al, 1994; Barger et al, 2005, 2006; Fortson, 2004; National Transportation Safety Board, 1990; Rajaratnam and Arendt, 2001; Spiegel et al, 1999; US Nuclear Regulatory commission, 1986).
Several countermeasures have been tested to minimize or avoid the decrements in performance that are related to sleepiness caused by prolonged wakefulness and working at adverse circadian phases. These have included behavioral (naps, exercise, work breaks), environmental (light), and pharmacological interventions. Of the latter group, amphetamines and caffeine have been extensively studied. Although amphetamines reduce sleepiness, they have been associated with addiction potential and troubling side-effects. Caffeine on the other hand is a widely used stimulant with validated efficacy that can, however, negatively affect subsequent sleep. To date, most countermeasures have been studied acutely in relatively short sleep deprivation protocols. Such protocols do not allow separate quantification of the influences of homeostatic sleep pressures which builds up with time awake, and circadian phase on performance and countermeasure effectiveness. Specifically, acute sleep deprivation protocols cannot determine whether a countermeasure acts on homeostatic (that is, sleep/wakefulness dependent) or circadian determinants of performance. In contrast, the previously reported forced desynchrony protocol dissociates the endogenous circadian rhythm from the sleep-wakefulness cycle, allowing quantification of the contribution of each of these factors to various parameters of interest (Boivin et al, 1997; Dijk et al, 1992; Johnson et al, 1992; Wyatt et al, 1999, 2004). In a study of repeated low-dose caffeine administration using a forced desynchrony protocol, it was shown that caffeine reduced some of the detrimental effects on performance related to homeostatic sleep pressure, but was less effective in counteracting the circadian contribution to performance degradation, and in addition impaired sleep efficiency (Wyatt et al, 2004).
Modafinil, 2-[(diphenylmethyl) sulfinyl] acetamide, is a wakefulness-promoting agent approved for use in the treatment of excessive sleepiness associated with narcolepsy, sleep apnea, and shift-work sleep disorder. This agent increases activity in hypothalamic arousal regions (Engber et al, 1998; Gallopin et al, 2004) and has shown efficacy in partially reversing performance impairment associated with simultaneous acute sleep deprivation and circadian misalignment (Bodenmann et al, 2009; Brun et al, 1998; Buguet et al, 1995; Caldwell et al, 2000; Czeisler et al, 2005; Pigeau et al, 1995; Wesensten et al, 2002). However, the durability of modafinil as a countermeasure to recurrent wakefulness extension is untested. Similarly, no extant study has evaluated the ability of modafinil to reduce the detrimental effects of working at an adverse circadian phase, separated from the effects of homeostatic sleep pressure.
Here, we present an analysis of the effects of modafinil on performance, alertness, sleep, and core body temperature in a double-blind, placebo-controlled trial.
PARTICIPANTS AND METHODS
Participants
Nineteen healthy adults, eight females and 11 males participated. One male participant withdrew on the second active-treatment day, due to possible side effects of treatment. Data from the remaining 18 participants (mean±SD age 23.9±4.0; range 18–30 years) are included in this report. All participants were screened for medical and psychiatric suitability based upon medical history, physical and psychological exams, blood and urine chemistries, and electrocardiogram. Toxicology screens for drug use verified that participants were drug free near the beginning of the screening process and upon admission to the laboratory. Participants each gave informed consent in writing. The protocol was approved by the Brigham and Women's Hospital/Partners Health Care System Human Research Committee.
Protocol
Experimental procedures
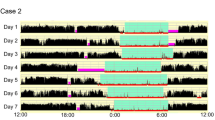
Participants maintained consistent sleep-wakefulness schedules with approximately 8 h of sleep for 3 weeks before admission, verified by call-in times to a time-stamped voice recorder, sleep logs, and for at least 1 week by wrist actigraphy (Minimitter, Sun River, OR, USA). Studies began with three 24-h baseline days with 8 h scheduled sleep to allow participants to acclimatize to the laboratory conditions. On days 4–28 of the 31-day inpatient protocol, participants were scheduled to a forced desynchrony protocol (Figure 1) for 14 consecutive 42.85-h ‘days’, each consisting of a 28.57-h episode of scheduled wakefulness and a 14.28-h episode of scheduled rest/sleep. Participants were scheduled to sleep in darkness and during scheduled wakefulness they were exposed to very dim room light (<15 lux maximum). The 42.85-h day length is known to be outside the range of entrainment of the human circadian pacemaker, that is, the circadian pacemaker cannot adapt to the 42.85-h day length and instead it continues to oscillate at its near 24-h intrinsic period (Czeisler et al, 1999; Wright et al, 2001). This allows wakefulness and sleep episodes to be distributed across a range of circadian phases throughout the inpatient stay and provides for relatively long wakefulness episodes.
Double Raster Plot of the study protocol. Days 1–3 and 30–31 were 24-h days with 16-h episodes of scheduled wakefulness and 8-h episodes of scheduled rest/sleep. Participants were studied in forced desynchrony during calendar days 4–29 consisting of fourteen 42.85-h days with of 28.57 h of scheduled wakefulness and 14.28 h of scheduled rest/sleep.
Participants lived in an environment free of time-cues, with no clocks, radios, newspapers, nor internet access. Participants were required to remain awake during scheduled wakefulness episodes and were required to stay in bed during scheduled rest/sleep episodes. Technicians monitored the participants from the control room and verbally awakened them after observing inadvertent sleep onsets. When participants were not performing assigned tasks, they were permitted to move about the suite, read, write, listen to music, watch videos, play video games, or converse with study staff. Room temperature was maintained at ∼24.5°C.
Drug treatment
Participants were treated according to a randomized double-blind placebo-controlled parallel-group design. Equal numbers of men and women were randomized to each treatment group. No significant differences in weight or BMI were observed between the treatment groups. All participants had a 2-day run-in of placebo-treatment single-blind, consisting of two tablets three times per day on days 2–3. During the forced desynchrony protocol, participants received modafinil or placebo double-blind, three times per wakefulness episode. Participants in the placebo-treatment group (N=9; five male participants, four female participants) received two placebo tablets at scheduled wake time and again after 9.58 and 19.16 h scheduled wakefulness. Participants in the modafinil-treatment group (N=9; five male participants, four female participants) received two tablets (placebo and 100 mg modafinil) at wake time and after 9.58 h scheduled wakefulness, and then two tablets (100 mg modafinil each) at 19.16 h scheduled wakefulness. Modafinil dosing was chosen to counteract the accumulation in homeostatic drive occurring across prolonged wakefulness episodes. In opposition to the increased homeostatic drive for sleep in the latter one-third of the wakefulness episode, we gave a dose equivalent to the dose used in a shift work disorder-clinical trial (Czeisler et al, 2005). The 200 mg dose was chosen because it has been shown to have significant beneficial effects on performance without deleterious effects on sleep.
Assays and Measurements
Temperature
Body temperature was measured every minute by means of a rectal thermistor (Yellow Springs Instruments, Yellow Springs, OH, USA), except during showers and bowel movements.
Performance tests
Participants performed a ∼30-min battery of neurobehavioral function tests every 2 h beginning 2 h after scheduled wake time. Cognitive-psychomotor performance were measured with the 3-min Digit Symbol Substitution Test (DSST) and a 4-min mathematical Addition Task (Dijk et al, 1992; Johnson et al, 1992). Short-term memory was measured with a 6-element word-pair Probed Recall Memory (PRM) task. Sustained attention was measured with the 10-min Psychomotor Vigilance Task (PVT), from which we assessed the means of the fastest 10% and slowest 10% of all reaction times, the median reaction times, and the number of lapses (that is, reaction times >500 ms)(Dinges et al, 1997). Fine motor control was assessed using the Trackball test (Eddy et al, 1998) with a slight modification adding random noise. Subjective sleepiness and alertness were measured with the Karolinska Sleepiness Scale (KSS) and with a visual analog scale (VAS), respectively. Attentiveness was also measured using the VAS. These tests were selected because they are known to vary with the circadian rhythm of body temperature and to be sensitive to sleep loss (Dijk et al, 1992; Dinges et al, 1997; Wright et al, 2002; Wyatt et al, 1999). In addition to the 2-hourly test batteries, the VAS and the KSS were administered every thirty minutes during scheduled wakefulness episodes.
Polysomnographic recording
Polysomnographic recordings were obtained during all sleep episodes with a digital recorder (Vitaport-3, TEMEC Instruments B.V., Kerkrade, The Netherlands), and included EEG (C3/A2, C4/A1, O1/A2, O2/A1), electrooculogram (EOG), and chin electromyogram (EMG). The signals were high-pass filtered [time constants: 0.68 s (EEG, EOG), or 0.015 s (EMG)], low-pass filtered [Bessel, 24 dB/octave; -6 dB at 70.1 Hz (EEG), 34.8 Hz (EOG), or 100.0 Hz (EMG)], and digitized [resolution: 12 bit, sampling rate: 256 Hz (EEG, EMG), or 128 Hz (EOG)]. Vigilance state scoring was performed on a 30-s basis according to standardized criteria (Rechtschaffen and Kales, 1968) by observers blind to condition. In addition, during roughly the 4th through the 27th hour of the scheduled wakefulness episodes, the EEG (Fz, Cz, Pz, Oz referenced to linked mastoids) and the electrooculogram (two derivations, right upper canthus and left lower canthus) were recorded (Vitaport-3, TEMEC Instruments B.V.) to quantify physiological sleepiness. The latter was determined in two ways: (1) by quantifying the 30-s epochs of wakefulness that contained at least one slow eye movement (SEM, identified as conjugate, sinusoidal deflections in the electrooculagrams unrelated to body movements; no amplitude criterion), and (2) by quantifying the 30-s epochs of inadvertent sleep, that is, the epochs that were scored as sleep based on conventional criteria (Rechtschaffen and Kales, 1968).
Data analysis
The intrinsic circadian period of the core body temperature rhythm was estimated in each participant using a non-orthogonal spectral analysis technique. That is, temperature data were fitted with periodic components corresponding to both the forced period of the imposed sleep-wakefulness cycle (42.85 h) and the sought-for period of the endogenous circadian temperature rhythm, together with their harmonics, using an exact maximum likelihood fitting procedure (Czeisler et al, 1999). Based on each individual's circadian period (eg, 24.3-h) and phase of the temperature rhythm, the neurobehavioral, sleep, slow eye movements, and temperature data were then averaged into six 60-degree circadian-phase bins with zero degrees corresponding to the fitted minimum of the endogenous circadian temperature cycle. The data were further averaged into six 4.76-h wake-bins according to the duration of scheduled wakefulness (neurobehavioral data, inadvertent sleep episodes during scheduled wakefulness, slow eye movement data). The duration of the previously scheduled sleep episode was averaged into six 2.38-h bins for sleep stage data. The temperature data were averaged across the entire 42.85-h forced desynchrony day into nine 4.76-h bins. Finally, to assess whether an effect of treatment group was observed across the forced desynchrony, we also binned the participants’ neurobehavioral data by scheduled wakefulness episode.
Statistical Analysis
Individual differences in neurobehavioral performance capability at baseline (except for the PVT) were minimized by expressing performance as change scores from baseline, by subtracting from each data point the mean performance obtained during the wakefulness episode of baseline day 3. Analysis of PVT data was performed on reciprocal median reaction times (1/median reaction time), reciprocal slowest 10% of responses (1/slowest 10% of responses), transformed lapses [√ lapses+√ (1+lapses)], and fastest 10% of responses (Bodenmann et al, 2009; Dinges et al, 1997). Epochs of inadvertent sleep were expressed as a percentage of the total number of recorded epochs within each wakefulness bin or circadian phase bin, whereas slow eye movements were expressed as a percentage of all epochs scored as wake within a bin. During the scheduled sleep episodes sleep efficiency was calculated.
For cognitive performance, alertness, and sleep parameters, dependent variables were analyzed using repeated measure ANOVAs with factors of drug-treatment group(DRUG), circadian phase [PHASE (degrees 0, 60, 120, 180, 240, 300)] and duration of previous scheduled wakefulness [WAKE (6 bins of 4.76-h each)] or, for sleep analysis, of previous scheduled sleep [SLEEP (6 bins of 2.38-h each)]. Temperature was analyzed using two-way ANOVAs with factors of drug-treatment group (DRUG) and scheduled wakefulness vs sleep (STATE), or drug-treatment group (DRUG) and circadian phase (PHASE). VAS ‘Attentive-Dreamy’ comparisons for the first two active treatment days were analyzed using repeated measure ANOVAs with factors of study day (DAY), drug-treatment group (DRUG), and duration of previous scheduled wakefulness (WAKE). Neurobehavioral performance across the forced desynchrony was analyzed using a two-way ANOVA with factors of drug-treatment group (DRUG) and scheduled wake period (SCHEDULED WAKE PERIO). Planned comparisons with f-test and modified Bonferroni correction factors were used to compare performance and physiological sleepiness data at each time awake and circadian phase bin.
RESULTS
Baseline Characteristics and Circadian Period
The participants’ circadian pacemakers were unable to entrain to the imposed 42.85-h light–dark cycle. Mean (±SD) estimates of the period of the intrinsic circadian rhythm derived from core body temperature were 24.11 (±0.19) for the placebo-treatment group and 24.23 (±0.23) for the modafinil-treatment group (NS; t-test p=0.5).
Temperature
A significant DRUG by STATE effect was observed for body temperature (p<0.05). Specifically, average body temperature was higher during scheduled wakefulness episodes and lower during scheduled sleep episodes in the modafinil-treated participants as compared with the placebo participants (Figure 2 e). No significant treatment effect was seen on circadian temperature variations.
Effect of modafinil on neurobehavioral function and core body temperature as derived from the forced desynchrony protocol (see Figure 1). Data are expressed as a function of both time since scheduled waking and circadian phase. Neurobehavioral parameters (a–d) and body temperature (e) were averaged across the fourteen 42.85-h forced desynchrony days and plotted in the left column as a function of time elapsed since scheduled wake-time and in the right column against circadian phase. (a) Addition task number of correct responses. (b) PVT-Slowest 10% of responses. (c) PVT-median reaction time. (d) PVT-lapses (trials with reaction times >500 ms). (e) Core body temperature (CBT). The placebo-treatment group is represented by open squares (□) and the modafinil-treatment group is represented by closed circles (•). The timing of modafinil or placebo administration is indicated in the lowest panel of the left column by a triangle (▴) with each triangle representing 100 mg of modafinil. The data represent mean values (±SEM). For the addition task, a value of 0 represents the mean value for each participant during the last baseline day before the forced desynchrony. PVT data are graphically represented without transformation; however, because the PVT data were not normally distributed, the statistical analyses on the PVT data were performed on transformed data, which are represented graphically in the Supplementary on-line materials. Baseline day 3 scores are provided in the results section of the text. Planned comparisons for individual circadian and hours awake bins were assessed using an f-test on the relevant data with significance levels indicated by an asterisk (*) whenever the p-value was <0.0417 (modified Bonferroni correction). Note, scales are inverted for PVT measures.
Performance
The participants’ performance on the final 24-h baseline day before the forced desynchrony (baseline day 3) was assessed for all cognitive parameters. Baseline day 3 scores averaged per treatment group were as follows (listed as placebo (±SEM) vs modafinil (±SEM)): Addition Task, number correct 57 (±2.7) vs 55 (±2.1); DSST, number correct 56 (±0.8) vs 63 (±0.8); PRM, number correct 3 (±0.3) vs 3.1 (±0.2); PVT, slowest 10% 534 (±40) vs 463 (±14) ms; PVT, median reaction time 295 (±8) vs 288 (±5.3) ms; and PVT, lapses 5.9 (±1.1) vs 3.7 (±0.6); PVT, fastest 10% 219 (±5) vs 215 (±3) ms; TRK, losses 10 (±1.8) vs 4 (±0.7); KSS 3.6 (±0.1) vs 3.6 (±0.1); VAS, alert 62 (±1.5) vs 65 (±1.4); VAS, attentive 67 (±1.6) vs 67 (±1.4). The only significant difference between treatment groups on baseline day 3 was seen for the DSST, number correct (p=0.05; all other comparisons p>0.05).
Statistical analyses of performance data during the forced desynchrony portion of the protocol are presented in the Table and performance parameters are graphically represented in Figure 2 and in the Supplementary materials section. As expected, all performance parameters deteriorated with advancing number of hours awake and near the circadian temperature minima (main effects for WAKE and PHASE; all p<0.01). Modafinil significantly improved performance on a cognitive-psychomotor performance task (number of correct responses on the Addition Task; main effect of DRUG, p=0.02; Figure 2a).
Significant DRUG by WAKE interactions showing better performance in modafinil-treated vs placebo-treated participants were observed for PVT-derived measures (slowest 10% of reaction times, median reaction times, and number of lapses; all p⩽0.01; Figure 2b–d, left column), the DSST (number of correct responses; p=0.03; Supplementary Figure S2a, left column), and for the fine motor control task (trackball number of control losses; p<0.01; Supplementary Figure S2c, left column). The performance-enhancing effects of modafinil were most apparent with increasing hours awake.
DRUG by PHASE interactions showing better performance in modafinil-treated vs placebo-treated participants were observed for the Addition Task (p<0.01) and PVT median reaction times (p<0.05; Figure 2a and c, right column); a trend for significance was seen for PVT fastest 10% reaction times (p=0.057; Figure S1 d, right column). These performance-enhancing effects of modafinil were most apparent in the bin just after the circadian temperature minimum, which is equivalent to the hours occurring just after habitual awakening. Modafinil had no significant effect on the performance of the Probed Recall Memory task.
Finally, a significant DRUG by SCHEDULED WAKE EPISO interaction showing progressive improvement in performance of the addition task across the forced desynchrony was observed in modafinil-treated, but not placebo-treated, participants (p<0.00005; Figure 6). Modafinil-treated participants performance was higher than baseline by wakefulness episode 4 of the forced desynchrony with continued improvement across the 14 forced desynchrony days whereas placebo-treated participants’ performance levels did not reach baseline during study. The other neurobehavioral parameters did not demonstrate similar improvement across the course of the study.
Modulation of inadvertent sleep episodes (a) and slow eye movements (b) during scheduled wakefulness episodes, and of sleep efficiency during scheduled sleep (c). In the left column, data are plotted as a function of time since scheduled waking (a, b) or of time since scheduled sleep (c); in the right column data are plotted as a function of circadian phase. Inadvertent sleep and slow eye movements during scheduled wakefulness were each scored on a 30-s basis. Inadvertent sleep was expressed as a percentage of the total number of recorded epochs within each wakefulness bin or circadian phase bin, whereas slow eye movements were expressed as a percentage of all epochs scored as wake within a bin. 0 degrees corresponds to the nadir of core body temperature. Sleep efficiency was calculated as the percentage of the total recording within a bin scored as sleep. Planned comparisons for individual circadian and hours awake bins were assessed using an f-test with significance levels indicated by an asterisk (*) whenever the p-value was <0.0417 (modified Bonferroni correction). The timing of modafinil or placebo administration is indicated in the lowest panel of the left column by a triangle (▴) with each triangle representing 100 mg of modafinil. The placebo-treatment group is represented by open squares (□) and the modafinil-treatment group is represented by closed circles (•). Data represent mean values (±SEM).
Modulation of subjective sleepiness as measured by the Karolinska Sleepiness Scale (KSS, a), alertness on the visual analog scale (VAS ‘Alert-Sleepy’, b), and attentiveness on the visual analog scale (VAS ‘Attentive-Dreamy’, c) plotted as a function of time since scheduled waking (left column) and circadian phase (right column). A value of zero represents the mean value for each participant during the last baseline day before the forced desynchrony. Baseline day 3 scores are provided in the results section of the text. Planned comparisons for individual circadian and hours awake bins were assessed using an f-test with significance levels indicated by an asterisk (*) whenever the p-value was <0.0417 (modified Bonferroni correction). The timing of modafinil or placebo administration is indicated in the lowest panel of the left column by a triangle (▴) with each triangle representing 100 mg of modafinil. The placebo-treatment group is represented by open squares (□) and the modafinil-treatment group is represented by closed circles (•). Data represent mean values (±SEM).
Attentiveness on the visual analog scale (VAS ‘Attentive-Dreamy’) plotted as a function of time since scheduled waking on the first and second active treatment days. A value of zero represents the mean value for each participant during the last baseline day before the forced desynchrony. The baseline day 3 scores are provided in the results section of the text. Higher scores represent higher alertness. The timing of modafinil or placebo administration is indicated by a triangle (▴) with each triangle representing 100 mg of modafinil. The placebo-treatment group is represented by open squares (□) and the modafinil-treatment group is represented by closed circles (•). Data represent mean values (±SEM).
Addition Task-number correct plotted as a function of the forced desynchrony wakefulness episodes. The data represent mean values (±SEM). A value of zero represents the mean value for each participant during the last baseline day before the forced desynchrony. The baseline day 3 scores are provided in the results section of the text. The placebo-treatment group is represented by open squares (□) and the modafinil-treatment group is represented by closed circles (•).
Sleepiness and Attentiveness
Occurrence of inadvertent sleep and slow eye movements during scheduled wakefulness episodes were used as objective measures of physiological sleepiness (see Table 1 and Figure 3a and b). During scheduled wakefulness episodes, placebo-treated participants were significantly more likely to show inadvertent sleep (2.2 vs 1.1%; p=0.03). A differential effect of treatment on inadvertent sleep appeared about 10-h after scheduled wake time and increased continuously thereafter during the 28.57 h wakefulness episode (DRUG by WAKE; p<0.01; Figure 3 a, left column). On the other hand, a decrease in inadvertent sleep was seen at all circadian phases in the modafinil-treated participants, but was largest shortly after the temperature minimum (DRUG by PHASE; p<0.01; Figure 3a, right column). However, no significant effect of treatment was seen in slow eye movements (Figure 3b).
Modafinil had no effect on subjective sleepiness, as assessed by KSS and VAS ‘Alert-Sleepy’ (Figure 4a and b). The VAS, with anchors ‘Attentive’ and ‘Dreamy’, (Figure 4 c) showed a trend for a main effect for DRUG (p=0.051) and a significant 3-way interaction (p<0.01; Figure 4c). The main effect for DRUG and the 3-way interaction, showed that the placebo-treatment group, reported higher ‘attentiveness’ across all WAKE and PHASE bins. Analysis of the first 2 days of active treatment suggests that this effect only became apparent on the second treatment day (Figure 5; DRUG by day; p=0.07).
Sleep
Sleep efficiency is represented in Figure 3c. No significant effect of drug treatment was observed for any sleep parameter [total sleep time, sleep efficiency, individual sleep stages, sleep latency, and wake after sleep onset (WASO)].
Adverse Events
No serious adverse events were observed. Two non-serious adverse events were reported to the IRB during the course of the study. The first participant complained of nausea during the first two placebo-treated baseline days and subsequently developed abdominal discomfort, a sore throat, loose stool, and worsened nausea during the first 2 days of active treatment with modafinil. On the second active-treatment day the attending physician judged his symptoms to be possibly related to the study medication and he was disempaneled. A second participant, who was a rock guitarist, reported that tinnitus, which he had experienced intermittently before study enrollment, worsened during and after the study. The attending physician judged his symptoms to be unrelated to the study medication. This participant was in the active-treatment group and completed the study.
DISCUSSION
Findings from this study show that 400 mg of modafinil, divided into three doses, enabled participants to better sustain wakefulness, alertness and performance throughout recurrent nearly 30-h wakefulness episodes, even at adverse circadian phases. Moreover, the effectiveness of modafinil as a countermeasure against the performance deterioration associated with both recurrent wakefulness extension and circadian misalignment was observed during 25 days of study. Of the currently measured parameters, modafinil's effects were most impressive on cognitive-psychomotor speed and sustained attention. Performance on the Addition Task was substantially improved at all hours of scheduled wakefulness and during all circadian phases. PVT lapses were halved by modafinil at adverse circadian phases and advanced hours awake, whereas the effects were smaller at other times. A sizable effect of modafinil on median reaction time was observed, with a substantial reduction of both circadian- and wake-dependent performance decrements as compared with the placebo-treatment group. Similar effects were seen with participants’ slowest 10% of responses on the PVT. Modafinil also showed efficacy in limiting performance impairments in a fine motor control task (Trackball-control losses). Some of these effects may be linked to the reduction in inadvertent sleep that was observed in the modafinil-treated participants during scheduled wakefulness.
This study extends the findings of previous investigations by showing modafinil's efficacy as a countermeasure to neurobehavioral impairments associated with recurrent wake extension in otherwise healthy participants. The nature of the forced desynchrony protocol, as described above, has allowed us to substantiate the efficacy of modafinil as a countermeasure to the neurobehavioral impairments associated with wakefulness at adverse circadian phases.
Though direct comparisons are difficult, modafinil's efficacy is at least comparable to that of repeated low-dose caffeine administration in a similar 42.85 h forced desynchrony protocol (Wyatt et al, 2004). Although caffeine was effective in counteracting the wake-dependent performance degradation, its effect on impairments associated with performing at an adverse circadian phase were more limited. This is consistent with caffeine's presumed mechanism of action as an antagonist to adenosine, the putative mediator of homeostatic sleep pressure (Dunwiddie and Masino, 2001; Landolt, 2008). Modafinil, on the other hand, showed more robust effects on both circadian- and wake-dependent influences on performance. This is also consistent with its known stimulatory effects on hypothalamic wake-promoting regions (Engber et al, 1998; Gallopin et al, 2004; Scammell and Matheson, 1998), structures that are under the influence of both the circadian pacemaker and sleep-promoting systems.
In addition, modafinil-treated participants' performance on the Addition Task improved progressively across the forced desynchrony protocol. Repeated administration of the Addition Task is known to be associated with learning, that is, improved cognitive performance across time, during circadian entrainment. We have previously shown that under conditions of sleep loss due to circadian misalignment that this type of learning is greatly attenuated, consistent with the results in the placebo-treated participants (Wright et al, 2006). This new finding suggests that modafinil treatment rescues learning in the setting of extended wakefulness and sleep loss induced by chronic circadian misalignment.
During scheduled wakefulness episodes, modafinil had no effect on slow eye movements but halved inadvertent sleep onsets across all circadian phases and with increasing hours awake. Modafinil did not negatively affect subjective alertness, as chronic caffeine administration did (Wyatt et al, 2004). On the other hand, modafinil-treated participants were subjectively less attentive (more dreamy). This effect appears to have begun on the second day of active treatment, immediately after the first post-treatment night of sleep. The significance of the observed findings on the ‘Attentive-Dreamy’ scale is not known. One possible explanation for this difference is the increased inadvertent sleep seen during scheduled wakefulness, which occurred twice as often in the placebo-treated participants as in the modafinil-treated participants and which may have benefitted the former. It is also possible that while both caffeine and modafinil are largely effective in preventing full transitions into sleep, the two drugs may keep an individual at different stages of the wake–sleep continuum. Feeling less attentive (modafinil) may correspond to an earlier, less severe stage of this continuum than feeling sleepy (caffeine). The observation that changes in subjective attentiveness only began to appear on the second active-treatment day suggests the possibility that modafinil affects sleep in a manner not detected with conventional sleep stage analysis. Findings from a recent study in humans showed no effect of modafinil (100 mg, administered 17 h before bedtime) on EEG power spectra during sleep (Bodenmann et al, 2009). On the other hand, a study in rats reported that the initial homeostatic rise of EEG power density within the range of slow-wave activity (0.75–4.0 Hz) during non-REM sleep was smaller following modafinil-induced wakefulness than following non-pharmacologically induced wakefulness. (Kopp et al, 2002). Thus, it will be interesting to investigate in future whether modafinil at a dose used in the current study can influence the expression of EEG slow-wave activity in sleep, particularly because this variable was suggested to affect some aspects of daytime functioning (Walsh et al, 2006; Aeschbach et al, 2008).
In contrast to repeated low-dose caffeine and amphetamines (Buguet et al, 1995; Wyatt et al, 1999), repeated modafinil administration produced no measurable deleterious effect on scheduled sleep. Sleep efficiency, sleep stages, total sleep time, wakefulness after sleep onset, and sleep latency did not differ between the two treatment groups. These results are consistent with earlier studies, including our own showing that 200 mg of modafinil given 9 h before bedtime did not result in sleep disturbance (Walsh et al, 2004; Czeisler et al, 2005). Also, within the rigid parameters of this study and contrary to a previous study, modafinil treatment did not result in a reduced repayment of sleep debt associated with recurrent wakefulness extension (Buguet et al, 1995). Modafinil treatment was generally well-tolerated in our study and no serious adverse events were observed. Two non-serious adverse events were seen, one of which was judged by the attending physician to be possibly related to the study medication and one of which was judged to be unrelated to the study medication.
Finally, modafinil treatment was associated with a differential effect on core body temperature according to behavioral state. Specifically, modafinil-treated participants had higher CBT during wakefulness and lower CBT during sleep than placebo-treated participants. A few studies have shown waking temperature elevation associated with modafinil use during acute sleep deprivation but have not shown a reciprocal fall in body temperature during recovery sleep (Brun et al, 1998; Pigeau et al, 1995). One study suggested that cold-challenged humans showed reduced heat production and augmented heat loss with modafinil use (Bourdon et al, 1994). The mechanism underlying the observations of the present study is not known but may be of relevance with regard to performance. Specifically, a direct relationship between higher CBT during wakefulness and better performance, after controlling for homeostatic and circadian effects, has been shown (Wright et al, 2002). The relative role that temperature elevation might have played in the performance of our participants is, however, uncertain.
In conclusion, our data confirm the efficacy of modafinil as a countermeasure to the neurobehavioral impairments associated with recurrent wakefulness extension and circadian misalignment. Unlike caffeine, modafinil also showed specific efficacy in its ability to offset performance impairment associated with functioning at an adverse circadian phase. Finally, our data show that chronic modafinil use did not appear to adversely affect either waking alertness, nor the quantity and quality of subsequent sleep. Our findings suggest that modafinil may be effective in mitigating some of the adverse effects on performance or safety associated with unavoidable wakefulness extension and work at adverse circadian phases. Given the recent recognition of biological factors that may contribute to individual vulnerability to circadian misalignment and extended wakefulness (Czeisler, 2009), development of targeted treatments for at-risk populations with wakefulness-promoting therapeutics may be an important strategy for improving safety in around-the-clock operations. Large-scale clinical research trials are needed to address this important public policy issue.
References
Aeschbach D, Cutler AJ, Ronda JM (2008). A role for non-rapid eye movement sleep homeostasis in perceptual learning. J Neurosci 28: 2766–2772.
Åkerstedt T, Czeisler CA, Dinges DF, Horne JA (1994). Accidents and sleepiness: a consensus statement from the international conference on work hours, sleepiness and accidents, Stockholm, 8-10 September 1994. J Sleep Res 3: 195.
Barger LK, Cade BE, Ayas NT, Cronin JW, Rosner B, Speizer FE, et al, Harvard Work Hours, Health and Safety Group (2005). Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med 352: 125–134.
Barger LK, Ayas NT, Cade BE, Cronin JW, Rosner B, Speizer FE et al (2006). Impact of extended-duration shifts on medical errors, adverse events, and attentional failures. PLoS Med 3: e487.
Bodenmann S, Xu S, Luhann UFO, Arand M, Berger W, Jung HH et al (2009). Pharmacogenetics of modafinil after sleep loss: catechol-o-methyltransferase genotype modulates waking functions but not recovery sleep. Clin Pharmacol Ther 85: 296–304.
Boivin DB, Czeisler CA, Dijk D-J, Duffy JF, Folkard S, Minors DS et al (1997). Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry 54: 145–152.
Bourdon L, Jacobs I, Bateman WA, Vallerand AL (1994). Effect of modafinil on heat production and regulation of body temperatures in cold-exposed humans. Aviat Space Environ Med 65: 999–1003.
Brun J, Chamba G, Khalfallah Y, Girard P, Boissy I, Bastuji H et al (1998). Effect of modafinil on plasma melatonin, cortisol and growth hormone rhythms, rectal temperature and performance in healthy subjects during a 36 h sleep deprivation. J Sleep Res 7: 105–114.
Buguet A, Montmayeur A, Pigeau R, Naitoh P (1995). Modafinil, d-amphetamine and placebo during 64 hours of sustained mental work. II. Effects on two nights of recovery sleep. J Sleep Res 4: 229–241.
Caldwell JA, Caldwell L, Smythe NK, Hall KK (2000). A double blind, placebo-controlled investigation of the efficacy of modafinil for sustaining alertness and performance of aviators: a helicopter simulator study. Psychopharmacology 150: 272–282.
Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW et al (1999). Stability, precision, and near-24-h period of the human circadian pacemaker. Science 284: 2177–2181.
Czeisler CA, Walsh JK, Roth T, Hughes RJ, Wright KP, Kingsbury L, et al, US Modafinil in Shift Work Sleep Disorder Study Group (2005). Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med 353: 476–486.
Czeisler CA (2009). Medical and genetic differences in the adverse impact of sleep loss on performance: ethical considerations for the medical profession. Trans Am Clin Climatol Assoc 120: 249–285.
Dijk D-J, Duffy JF, Czeisler CA (1992). Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res 1: 112–117.
Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE et al (1997). Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 h per night. Sleep 20: 267–277.
Dunwiddie TV, Masino SA (2001). The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24: 31–55.
Eddy DR, Schiflett SG, Schlegel RE, Shehab RL (1998). Cognitive performance aboard the life and microgravity spacelab. Acta Astronaut 43: 193–210.
Engber TM, Koury EJ, Dennis SA, Miller MS, Contreras PC, Bhat RV (1998). Differential patterns of regional c-Fos induction in the rat brain by amphetamine and the novel wakefulness-promoting agent modafinil. Neurosc Lett 241: 95–98.
Fortson KN (2004). The diurnal pattern of on-the-job Injuries. Monthly Labor Review 127: 18–25.
Gallopin T, Luppi P-H, Rambert FA, Frydman A, Fort P (2004). Effect of the wake-promoting agent modafinil on sleep-promoting neurons from the ventrolateral preoptic nucleus: an in vitro pharmacologic study. Sleep 27: 19–25.
Hull JT, Wright Jr KP, Czeisler CA (2003). The influence of subjective alertness and motivation on human performance independent of circadian and homeostatic regulation. J Biol Rhythms 18: 329–338.
Johnson MP, Duffy JF, Dijk D-J, Ronda JM, Dyal CM, Czeisler CA (1992). Short-term memory, alertness, and performance: a reappraisal of their relationship to body temperature. J Sleep Res 1: 24–29.
Kopp C, Petit J-M, Magistretti P, Borbely AA, Tobler I (2002). Comparison of the effects of modafinil and sleep deprivation on sleep and cortical EEG spectra in mice. Neuropsychopharmacology 43: 110–118.
Landolt HP (2008). Sleep homeostasis: A role for adenosine in humans? Biochem Pharmacol 75: 2070–2079.
National Transportation Safety Board (1990). Marine accident report-grounding of the US tankship EXXON VALDEZ on Bligh Reef, Prince William Sound, near Valdez, Alaska, March 24. National Transportation Safety Board: Washington, DC. NTSB/MAR-90/04. 1989.
Pigeau R, Naitoh P, Buguet A, McCann C, Baranski J, Taylor M et al (1995). Modafinil, d-amphetamine and placebo during 64 h of sustained mental work. I. Effects on mood, fatigue, cognitive performance, and body temperature. J Sleep Res 4: 212–228.
Rajaratnam SMW, Arendt J (2001). Health in a 24-h society. Lancet 358: 999–1005.
Rechtschaffen A, Kales A (1968). A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. US Government Printing Office: Washington, DC.
Santhi N, Aeschbach D, Horowitz TS, Czeisler CA (2008). The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms 24: 341–352.
Scammell TE, Matheson J (1998). Modafinil: a novel stimulant for the treatment of narcolepsy. Exp Opin Investig Drugs 7: 99–112.
Spiegel K, Leproult R, van Cauter E (1999). Impact of sleep debt on metabolic and endocrine function. Lancet 354: 1435–1439.
US Nuclear Regulatory Commission (1986). Report on the Accident at the Chernobyl Nuclear Power Station. US Government Printing Office: Washington, DC.
Viola AU, Archer SN, James LM, Groeger JA, Lo JCY, Skene DJ et al (2007). PER3 polymorphism predicts sleep structure and waking performance. Curr Biol 17: 613–618.
Walsh JK, Randazzo AC, Stone KL, Schweitzer PK (2004). Modafinil improves alertness, vigilance, and executive function during simulated night shifts. Sleep 27: 434–439.
Walsh JK, Randazzo AC, Stone K, Eisenstein R, Feren SD, Kajy S et al (2006). Tiagabine is associated with sustained attention during sleep restriction: evidence for the value of slow-wave sleep enhancement? Sleep 29: 433–443.
Wesensten NJ, Belenky G, Kautz MA, Thorne DR, Reichardt RM, Balkin TJ (2002). Maintaining alertness and performance during sleep deprivation: modafinil versus caffeine. Psychopharmacology 159: 238–247.
Wright Jr KP, Hughes RJ, Kronauer RE, Dijk D-J, Czeisler CA (2001). Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci USA 98: 14027–14032.
Wright Jr KP, Hull JT, Czeisler CA (2002). Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol 283: R1370–R1377.
Wright Jr KP, Hull JT, Hughes RJ, Ronda JM, Czeisler CA (2006). Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci 18: 508–521.
Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk D-J (1999). Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol 277: R1152–R1163.
Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk D-J (2004). Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep 27: 374–381.
Acknowledgements
Research supported by US AFOSR F49620-00-1-0266. This study was conducted in the General Clinical Research Center (GCRC, supported by NIH Grant NCRR-GCRC-M01-RR02635) of the Brigham and Women's Hospital. This study also received financial support from Cephalon Inc. for the scoring of inadvertent sleep and slow eye movements in the scheduled wakefulness episodes. We thank Eymard Riel (RPSGT), Gregg Renchkovsky, Anna Leavitt, Laurel Fulham, and Brandon Lockyer (RPSGT) for scoring and overall polysomnography support. We thank the staff of the GCRC and the Chronobiology Core, under the direction of Dr Jeanne Duffy, for participant care and for carrying out the study protocol, as well as KC Malvey for assistance with the study coordination and participant recruitment. Special thanks are extended to Claude Gronfier PhD for assistance with data preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Grady has no conflict of interest to declare. Dr Aeschbach has received research support from NIH, the National Alliance for Research on Schizophrenia and Depression, and Cephalon Inc., and has served as a paid member of the scientific advisory board of Zeo Inc. Dr Wright has received research support from, has consulted for, and has participated in speaking engagements for Cephalon and Takeda; has consulted for Novartis, City of Boulder Public Works-Transportation and Utilities Cold Weather Operations, City of Boulder Fire-Rescue-Division of Emergency Services, Harvard Medical School-Teachers Institute, NASA Jet Propulsion Laboratory, and California Institute of Technology; and serves on the advisory board of and has received stock options from Zeo Inc. (formerly Axon Labs). Dr Czeisler has received consulting fees from or served as a paid member of scientific advisory boards for: Actelion Ltd.; Bombardier; Boston Celtics; Cephalon Inc.; Columbia River Bar Pilots; Delta Airlines; Eli Lilly and Co; Fedex Kinko's; Federal Motor Carrier Safety Administration (FMCSA), US Department of Transportation; Fusion Medical Education, LLC; Garda Síochána Inspectorate (Dublin, Ireland); Hypnion Inc.; Global Ground Support; Johnson & Johnson; Koninklijke Philips Electronics, NV; The Minnesota Timberwolves; Morgan Stanley; Norfolk Southern; Sanofi-Aventis Groupe; Portland Trail Blazers; Philips; Respironics Inc.; Sepracor Inc.; Sleep Multimedia Inc.; Sleep Research Society (for which Dr Czeisler served as president); Somnus Therapeutics Inc.; Takeda Pharmaceuticals; Vanda Pharmaceuticals Inc.; Vital Issues in Medicine; and Zeo Inc. Dr Czeisler owns an equity interest in Lifetrac Inc.; Somnus Therapeutics Inc.; Vanda Pharmaceuticals Inc., and Zeo Inc., and received royalties from McGraw Hill, The New York Times, and Penguin Press. Dr Czeisler has received lecture fees from the Accreditation Council of Graduate Medical Education; Alfresa; the American Academy of Allergy, Asthma and Immunology Program Directors; American Physiological Society; Association of University Anesthesiologists; Baylor College of Medicine; Beth-Israel Deaconess Medical Center; Brown Medical School/Rhode Island Hospital; Cephalon Inc.; Clinical Excellence Commission (Australia); Dalhousie University; Duke University Medical Center; Harvard University; Institute of Sleep Health Promotion (NPO); London Deanery; Morehouse School of Medicine; Mount Sinai School of Medicine; National Emergency Training Center; National Institutes of Health; North East Sleep Society; Osaka University School of Medicine; Partners HealthCare Inc.; Sanofi-Aventis Inc.; St Lukes Roosevelt Hospital; Takeda; Tanabe Seiyaku Co Ltd.; Tokyo Electric Power Company (TEPCO); University of Michigan; University of Pennsylvania; University of Pittsburgh; University of Tsukuba; University of Virginia Medical School; University of Wisconsin Medical School; World Federation of Sleep Research and Sleep Medicine Societies. Dr Czeisler has also received research prizes with monetary awards from the American Academy of Sleep Medicine; American Clinical and Climatological Association; Association for Patient-Oriented Research; National Institute for Occupational Safety and Health; National Sleep Foundation; and Sleep Research Society; clinical trial research contracts from Cephalon Inc., Merck & Co. Inc., and Pfizer Inc.; an investigator-initiated research grant from Cephalon Inc.; and his research laboratory at the Brigham and Women's Hospital has received unrestricted research and education funds and/or support for research expenses from Cephalon Inc., Koninklijke Philips Electronics, NV, ResMed, and the Brigham and Women's Hospital. The Harvard Medical School Division of Sleep Medicine (HMS/DSM), which Dr Czeisler directs, has received unrestricted research and educational gifts and endowment funds from: Boehringer Ingelheim Pharmaceuticals Inc, Cephalon Inc., George H Kidder, Esq., Gerald McGinnis, GlaxoSmithKline, Herbert Lee, Hypnion, Jazz Pharmaceuticals, Jordan's Furniture, Merck & Co. Inc., Peter C Farrell, PhD, Pfizer, ResMed, Respironics Inc., Sanofi-Aventis Inc., Sealy Inc., Sepracor Inc., Simmons, Sleep Health Centers LLC, Spring Aire, Takeda Pharmaceuticals and Tempur-Pedic. The HMS/DSM has received gifts from many outside organizations and individuals including: Axon Sleep Research Laboratories Inc., Boehringer Ingelheim Pharmaceuticals Inc., Catalyst Group, Cephalon Inc., Clarus Ventures, Eli Lilly and Co., Farrell Family Foundation, Fisher & Paykel Healthcare Corporation, George H Kidder, Esq., GlaxoSmithKline, Hypnion Inc., Jordan's Furniture, Merck Research Laboratories, Park Place Corporation, Respironics Inc., Sanofi-Aventis Inc., Select Comfort Corporation, Sepracor Inc., Sleep Health Centers LLC, Takeda Pharmaceuticals, Tempur-Pedic Medical Division, Total Sleep Holdings, Vanda Pharmaceuticals Inc. The HMS/DSM Sleep and Health Education Program has received Educational Grant funding from Cephalon Inc., Takeda Pharmaceuticals, Sanofi-Aventis Inc. and Sepracor Inc. Dr Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon Inc. and holds a number of process patents in the field of sleep/circadian rhythms (eg, photic resetting of the human circadian pacemaker). Since 1985, Dr Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Grady, S., Aeschbach, D., Wright, K. et al. Effect of Modafinil on Impairments in Neurobehavioral Performance and Learning Associated with Extended Wakefulness and Circadian Misalignment. Neuropsychopharmacol 35, 1910–1920 (2010). https://doi.org/10.1038/npp.2010.63
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2010.63
Keywords
This article is cited by
-
Effects of total sleep deprivation on performance in a manual spacecraft docking task
npj Microgravity (2024)
-
Desynchronizing the sleep–wake cycle from circadian timing to assess their separate contributions to physiology and behaviour and to estimate intrinsic circadian period
Nature Protocols (2023)
-
Young adults are more vulnerable to chronic sleep deficiency and recurrent circadian disruption than older adults
Scientific Reports (2018)
-
Wake-Promoting Pharmacotherapy for Psychiatric Disorders
Current Psychiatry Reports (2014)