Abstract
Studies of procedural learning in medicated schizophrenia patients using predictive saccade paradigms have consistently demonstrated hypometric predictive responses. Findings from antipsychotic-naive schizophrenia patients indicate fewer or no deficits. This pattern of findings suggests that antipsychotic medications might adversely affect frontostriatal systems supporting procedural learning on this task. The accuracy and latency of predictive saccades were assessed in 25 antipsychotic-naive first-episode schizophrenia patients and 22 matched healthy individuals. Patients were retested after 6 weeks of treatment with risperidone. Healthy individuals were reevaluated after a similar time period. The ability to learn to time response initiation in anticipation of target appearance (target prediction) was not impaired in patients before or after treatment. In contrast, although no deficits were evident before treatment initiation, after treatment patients showed a marked decrease in the accuracy of predictive but not sensory-guided responses. The findings from pretreatment testing indicate that procedural learning is a relatively unaffected cognitive domain in antipsychotic-naive first-episode schizophrenia. Although treatment-emergent extrapyramidal symptoms were minimal, these data suggest that D2 antagonism in striatum after risperidone treatment was sufficiently robust to disrupt the generation of planned volitional behavior guided by internalized representations.
Similar content being viewed by others
INTRODUCTION
Cognitive dysfunction has long been established as a cardinal feature of schizophrenia (Bleuler, 1952; Kraepelin, 1925). Oculomotor studies are a promising approach for investigating these neurocognitive deficits and the effects of pharmacotherapy on their respective functional brain systems. The neural systems involved in the cognitive control of eye movements are well characterized through studies with nonhuman primates (Everling and Munoz, 2000), clinical studies of patients with focal lesions (Guitton et al, 1985; Pierrot-Deseilligny, 1994), and functional neuroimaging studies (Sweeney et al, 1996).
The predictive saccade paradigm examines ‘anticipatory’ behaviors, which are responses guided by learned internal representations about predictable environmental events (Simo et al, 2005). In this paradigm, a target stimulus typically shifts back and forth between two locations at a constant time interval as participants track the target with saccadic eye movements. The predictive saccade paradigm is thus a serial reaction time task assessing procedural learning, which is the ability to acquire a motor routine via repeated exposure to a task governed by invariant rules (Cohen et al, 1985).
In contrast to many procedural learning tasks that take hours or days to train participants to peak performance, healthy individuals begin to initiate predictive eye movements in anticipation of target appearance after only a few trials (ie after less than 10 s) and approach peak performance within 1 min. Thus, the task provides an efficient approach for evaluating procedural learning in clinical studies.
Using fMRI, we previously demonstrated that predictive saccades rely upon frontostriatal circuitry including dorsolateral prefrontal cortex (PFC), dorsomedial thalamus, and dorsal striatum, as well as the hippocampus and anterior cingulate cortex, in contrast to visually guided saccades that rely on sensorimotor systems (Simo et al, 2005). Because dorsal-striatal systems are affected by the D2 blockade associated with antipsychotic medications, studying predictive saccades can be informative about systems level effects of antipsychotic treatments.
Previous studies investigating predictive saccades in schizophrenia all documented hypometric (ie undershooting) predictive saccades in medicated, typically chronic schizophrenia patients (Crawford et al, 1995a; Hommer et al, 1991; McDowell et al, 1996; Thaker et al, 1996). Findings with regard to the ability to learn to accurately time predictive saccades in relation to stimulus appearance have been less consistent. McDowell et al (1996) reported faster response latencies, Thaker et al (1996) reported slower latencies, and Crawford et al (1995a) found no latency differences between schizophrenia and healthy groups. In a combined group of antipsychotic-free and antipsychotic-naive schizophrenia patients, Krebs et al (2001) reported hypometric predictive saccades, as did Hutton et al (2001) in a group of never-medicated patients. In contrast, Crawford et al (1995b) and Hommer et al (1991) reported no reductions in saccade accuracy in previously treated but medication-free patients. Thus, although medicated patients have consistently been shown to produce hypometric predictive saccades, the findings have been less consistent in medication-free patients.
The present study was designed to assess procedural learning in antipsychotic-naive schizophrenia patients, and the early effects of antipsychotic treatment on task performance, by administering the predictive saccade task to antipsychotic-naive first-episode patients before and 6 weeks after treatment with the second-generation antipsychotic risperidone. On the basis of results of existing studies with untreated and medicated schizophrenia patients, and the D2 receptor antagonism in the striatum associated with risperidone treatment, we predicted a significant decline in accurate predictive behavior in patients after treatment initiation.
MATERIALS AND METHODS
Participants
Written informed consent for all study procedures was obtained from 25 antipsychotic-naive adult in- and outpatients meeting DSM-IV criteria for schizophrenia according to the Structured Clinical Interview (SCID; First et al, 1995), and collateral clinical data were reviewed at consensus diagnosis meetings. Diagnoses were confirmed at 6-month follow-up visits as part of the prospective longitudinal study of first-episode psychoses in Pittsburgh. All patients were experiencing their first lifetime psychotic episode and had never been treated with antipsychotic medication. Any such patient presenting to the inpatient or outpatient services of the University of Pittsburgh Medical Center was informed of the study by a clinician. Of the forty-three patients who were screened, three (7.0%) refused participation, one (2.3%) was too psychotic/agitated to provide consent, and seven (16.3%) met exclusion criteria (one had a prior treatment history with antipsychotic medication, one did not meet diagnostic criteria, and five presented with current substance use problems). Of the remaining thirty-two patients who consented to study participation, three patients were judged by clinicians to be too psychotic to participate and to require immediate medication treatment, three patients refused participation after giving informed consent, and one patient later admitted to cannabis abuse. The remaining 25 patients participated in the study. The study protocol was approved by the Institutional Review Board of the University of Pittsburgh. All study participants gave written informed consent for all study procedures.
Clinical ratings were obtained by raters without knowledge of task performance. Ratings included the Brief Psychiatric Rating Scale (Overall and Gorham, 1962), the Schedule for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984a), the Schedule for the Assessment of Negative Symptoms (SANS; Andreasen, 1984b), the 24-item Hamilton Depression Rating Scale (Hamilton, 1960), and the Extrapyramidal Side Effects scale (EPSEs; McEvoy et al, 1991). A total of 22 healthy individuals, who did not meet criteria for any present or past Axis I disorder according to SCID interviews, were recruited from the surrounding community via advertisements. This group matched the patient group on age, gender, and estimated intellectual potential using a test of vocabulary knowledge (Ammons’ Quick Test; Ammons and Ammons, 1962; Table 1).
All participants met the following criteria: (1) age between 18 and 45 years; (2) no known systemic or neurologic disease, including seizures; (3) no history of head trauma with loss of consciousness; (4) no lifetime history of substance dependence or substance abuse within 3 months prior to study participation; (5) no benzodiazepines (five half-lives) prior to testing; (6) no prior treatment with electroconvulsive therapy; and (7) no coffee, tea, or cigarettes 1 h prior to testing.
Patients’ baseline eye movement studies and neuropsychological testing were conducted prior to treatment initiation. Treatment with the second-generation antipsychotic risperidone was started following baseline assessments, and follow-up testing was performed approximately 6 weeks after treatment initiation. Extrapyramidal side effects (EPS) were modest at the 6-week retesting (Table 1), but were sufficient in five patients to require low-dose (1–3 mg) benztropine. In addition, four patients also received antidepressant medication and one patient received lithium at the 6-week follow-up, including three of the benztropine-treated patients. No other medications were administered during the study period. Healthy individuals were studied over a similar interval.
Eye Movement Studies
Participants were seated in a darkened black room free from extraneous stimuli facing a circular black arc with a 1 m radius containing red light-emitting diodes (LEDs) embedded in the horizontal plane at eye level. The LEDs subtended approximately 0.2° of visual angle and were not visible unless illuminated. A chin and forehead rest minimized head movement. Participants were given no instructions to indicate that the stimulus sequence was predictable and were told only to look to the lights as they appeared.
Saccades were recorded using DC electrooculography (EOG; Grass Neurodata 12 Acquisition System, Astro-Med Inc., West Warwick, RI), and blinks were monitored using electrodes placed above and below the left eye. All recordings were digitized at 500 Hz (DI-210 14-bit A/D, DATAQ Instruments) and stored for offline analyses. Recordings were analyzed using custom software developed in our laboratory.
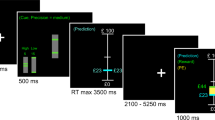
Predictive Saccade Task
The predictive saccade paradigm is a simple serial reaction time task in which individuals shift gaze between two target positions sequentially illuminated at a fixed temporal interval. Participants very quickly learn to anticipate target appearance, so that an increasing percentage of saccades are made close to target appearance on the basis of internally generated predictions about target appearance rather than actual visual stimulus appearance. For this study, participants looked toward visual targets alternating between two locations at 7.5° of visual angle to the left and right of central fixation. The target shifted between the two locations every 1.5 s (0.33 Hz) 10 times (ie 20 target presentations). There was no gap or overlap as new targets appeared contemporaneously as previous targets were extinguished. The latency (time from appearance of target to response initiation) and gain (proportion of distance moved to the target location) of primary saccades toward target locations were measured. To avoid confusion with small saccades made during ongoing fixation of targets, we defined the primary saccade as the first saccade toward the next target with a gain of at least 0.2 (ie a saccade on the order of 3° of visual angle).
Eye Data Analysis
Eye position recordings obtained during fixation of targets during each trial were used to convert voltage recordings to eye position in degrees of visual angle. This minimized artifacts resulting from EOG signal drift over the course of testing. Recordings from each trial were reviewed to identify primary saccades, artifacts (eg blinks and signal clipping), and occasional failures of software algorithms to correctly identify saccades that were then marked manually. Before analysis, digitized eye movement signals were smoothed using linear phase, finite impulse response low-pass filters.
Neuropsychological Assessments
All participants also underwent comprehensive neuropsychological testing in parallel with eye movement testing, as described in detail elsewhere (Hill et al, 2004). For the purposes of the present study, measures of psychomotor abilities, specifically finger tapping and grooved pegboard test scores, were examined in relation to predictive saccade performance (Table 2).
Statistical Analyses
There were no significant saccade by direction effects or group by direction interaction effects. Therefore, data from leftward and rightward saccades were pooled for analyses. To examine the change in saccade latency over the course of the task, primary saccades in the 19 trials (response to the first unpredictable target displacement was excluded) were collapsed into three blocks (block 1, trials 1–6; block 2, trials 7–12; and block 3, trials 13–19).
Performance on this task has both quantitative (eg response latency and accuracy) and qualitative dimensions (internally generated predictive responses vs sensory-driven visually elicited responses). To examine the qualitative aspects of performance, each primary saccade was classified as follows: sensory-guided saccades (eg visually elicited saccades) with latencies greater than 140 ms; predictive saccades with latencies less than 90 ms; and an indeterminate/intermediate group of speeded saccades with latencies between 90 and 140 ms. The rationale for this classification is threefold. First, sensory-guided (or visually elicited) saccades in no-gap paradigms (old target extinguishes contemporaneously with new target appearance) such as the one used in this study very rarely occur with latencies shorter than 140 ms (Becker, 1989; Fischer et al, 1993). Second, oculomotor studies generally classify saccades with latencies less than 100 ms as predictive because this reflects the minimal time necessary for perceiving a visual stimulus and performing sensorimotor transformations needed to initiate a motor response (Becker, 1989; Wenban-Smith and Findlay, 1991). We classified eye movements with latencies less than 90 ms as anticipatory movements. Third, whether saccades with latencies between 90 and 140 ms were predictive or not is difficult to determine, so they were considered separately as an indeterminate/intermediate group.
RESULTS
Latency of Saccades
At baseline, patients and healthy individuals demonstrated a significant reduction in saccade latencies across the three blocks of trials as they performed the predictive saccade task, reflecting an ability to learn the response sequence and to initiate saccades based on predictions about future target appearance (F(2, 44)=6.28, p<0.01). Also, the rate of learning to anticipate target appearance over trials, reflected in saccade latency reductions, did not differ between patients and healthy individuals (F(2, 44)=0.55, p=0.58; Figures 1 and 2a). Furthermore, treatment did not alter patients’ capacity to learn to time the initiation of saccades in anticipation of target appearance, as reflected in similar reduction in response latencies across blocks of trials from baseline to follow-up for patients and healthy individuals (F(2, 44)=0.27, p=0.76; Figure 2b).
Cumulative percentage of trial-wise saccade latencies. (a) The comparison of patients (triangles) and healthy individuals (circles) at the baseline evaluation. Note the nearly overlapping distributions of the response latency data. (b) The stability of patients’ response latencies from the baseline visit (solid line) to the 6-week follow-up. Predictive saccades had latencies less than 90 ms, speeded saccades had latencies between 90 and 140 ms, and sensory-guided saccades had latencies greater than 140 ms.
Accuracy of Saccades
The accuracy of patients’ sensory-guided saccades (latencies>140 ms) was comparable to that of healthy individuals (F(1, 43)=1.04, p=0.32; Figure 3a), and there was no group difference in change between baseline and follow-up testing in these responses (F(1, 43)=0.32, p=0.57). Findings were the same for speeded saccades (latencies between 90 and 140 ms) (F(1, 36)=2.06, p=0.16; Figure 3b for group differences) and (F(1, 36)=1.46, p=0.23 for differential change over time between groups). The accuracy of patients’ predictive saccades (latencies <90 ms), however, was significantly reduced after treatment relative to healthy individuals (F(1, 34)=10.98, p=0.002; Figure 3c). Although patients’ predictive saccades were accurate at baseline (t(40)=0.43, p=0.67), they were significantly less accurate (ie more hypometric) than healthy individuals at the 6-week follow-up (t(35)=4.35, p<0.001). Relative to baseline, the gain of patients’ saccades was reduced by 27% at follow-up (t(18)=−5.43, p<0.001), but healthy individuals’ saccade gain increased minimally by 8% from baseline to follow-up (t(16)=0.81, p=0.43). Exclusion of the five patients taking benztropine and the four patients taking antidepressants at the 6-week follow-up in secondary analyses did not change findings for saccade latencies or accuracy.
Mean (SE) error of saccade gain for patients and healthy individuals at baseline and 6-week follow-up. Patients’ saccade gain of sensory-guided (a) and speeded saccades (b) were comparable to those of healthy participants at both time points. There was a substantial decline in the gain of patients’ predictive saccades (c) at the 6-week follow-up relative to healthy individuals. **p<0.001.
Relationships with Clinical Ratings, Medication Dosage, and Neuropsychological Data
There were no significant associations between changes in the accuracy of sensory-guided, speeded or anticipatory saccades from baseline to 6-week follow-up and changes in clinical symptom ratings, risperidone dose, or EPS ratings. Associations between changes from baseline to follow-up of oculomotor and neuropsychological measures were minimal (Table 3).
DISCUSSION
The results of the present study demonstrate that antipsychotic-naive schizophrenia patients have the ability to use internal spatial and temporal representations to rapidly learn to perform a simple procedural learning task. Before treatment, schizophrenia patients learned the invariant spatiotemporal characteristics of target positions, created a motor program based on internally generated predictions about the learned sequence, and executed anticipatory behavior as quickly and accurately as healthy individuals. After treatment with risperidone, the ability to time saccadic eye movements to predictable target appearance was unchanged. However, although the accuracy of sensory-guided responses remained unchanged, the accuracy of predictive saccades, voluntary saccades made before sensory input can be used to guide responses, was significantly reduced. Thus, although the temporal aspect of predicting target appearance remained unchanged, either spatial representational memory or the ability to use it to guide voluntary action was significantly impaired after 6 weeks of risperidone therapy. This effect was not related to minimal treatment-emergent EPS or to changes in manual motor control, suggesting that the ability to generate accurate behavioral responses based on internal representations without sensory feedback is a process adversely and selectively impacted by risperidone treatment.
The demonstration of reduced accuracy of predictive saccades after treatment is consistent with findings from prior studies with medicated patients (Crawford et al, 1995a; Hommer et al, 1991; McDowell et al, 1996; Thaker et al, 1996). However, the present findings are the first to document the onset of this robust deficit after the initiation of antipsychotic therapy. Our results are also important in documenting that antipsychotic-naive schizophrenia patients do not have deficits on this procedural learning task, which stands out as an area of intact neurocognitive function in the context of widespread neuropsychological (Bilder et al, 2000; Hill et al, 2004) and oculomotor deficits (Clementz et al, 1994; Harris et al, 2006; Lencer et al, 2000; Ross et al, 1997; Sweeney et al, 1998).
Notably, our oculomotor findings with regard to intact procedural learning prior to treatment stand in contrast to those from studies of manual serial reaction time tasks and manual pursuit rotor and mirror-drawing studies. On all of these tasks, schizophrenia patients were impaired even before antipsychotic drugs were available (Huston and Shakow, 1948). The basis of impaired procedural learning on manual but not oculomotor skills remains to be clarified. This could be due to the typically greater level of task difficulty in manual tasks used previously, or to differential perturbation of the functional brain systems supporting the intentional control of manual and eye movements. Regardless, the absence of deficits on oculomotor procedural learning tests indicates that the central processes associated with anticipation and prediction that are needed to support spatial representations and temporal interval timing for procedural learning on predictive saccade tasks do not appear to be impaired in the disorder.
Some previous investigations have reported hypometric predictive saccades in untreated patients, such as Krebs et al (2001) who reported this finding in a combined group of medication-naive and medication-free patients. Our work differs from that study in that all of our patients were antipsychotic-naive at baseline, and thus were not affected by potential residual effects of prior antipsychotic treatment. Hutton et al (2001) reported hypometric saccades in drug-naive schizophrenia patients, which is not consistent with our observations. The reasons for the difference between the results are not clear, but methodological factors may be important. Hutton et al (2001) employed auditory as well as visual stimuli to cue shifts in target locations, and used a shorter interstimulus interval (1 s).
We propose three mechanisms that may account for the reduction in predictive saccade accuracy after treatment with risperidone: (1) a disruption in motor learning or voluntary motor control, (2) a disturbance of spatial working memory, and (3) a disruption in spatial mapping and memory.
Motor Learning and Control
Functional neuroimaging studies with healthy individuals indicate central roles for the striatum and frontal cortex in procedural learning (Krebs et al, 1998; Poldrack et al, 2005; Zedkova et al, 2007), consistent with human lesion and disease models (Salmon and Butters, 1995) and unit recording studies of behaving monkeys (Hikosaka et al, 1999). Kumari et al (2002) have shown reduced activation in frontostriatal circuitry in treated schizophrenia patients performing a procedural learning task. Further, Kumari et al (1997) reported an adverse effect of haloperidol and a facilitative effect of D-amphetamine on procedural learning in healthy participants, demonstrating a key role of dopamine in human procedural learning. Therefore, the impairments we observed in predictive saccade accuracy after antipsychotic treatment might be due to the effects of D2 antagonism in striatum or frontal cortex. Consistent with this hypothesis, Bedard et al (2000) and Purdon et al (2003) have reported adverse effects of risperidone treatment on procedural learning in schizophrenia. Kern et al (1998) did not observe a differential effect of risperidone vs haloperidol treatment on a manual sequence learning test, suggesting that drug induced changes in procedural learning may occur with both first- and second-generation antipsychotic medications.
In addition to planning and enacting motor responses without sensory guidance, the ability to estimate temporal intervals is also required to perform predictive saccade tasks. Mechanisms supporting temporal interval timing in the basal ganglia are also known to be dopamine dependent (Garraux et al, 2005; Hinton and Meck, 1997). Dopamine, via pars compacta projections to the striatum, modulates ‘clock’ speed of the striatal timing system (Hinton and Meck, 1997). Thus, the absence of treatment effects on the timing of responses, consistent with previous findings on a manual task (Green et al, 1997), suggests that timing systems in the striatum are not adversely affected by antipsychotic treatment. The ability to learn to time responses appropriately after treatment suggests that the treatment-emergent reduction in predictive saccade accuracy is not a result of disturbances in pars compacta input to the striatum, or in striatocerebellar circuits that are also important for response timing (Hikosaka et al, 1999). Rather, disturbances in frontostriatal integration may be a more likely mechanism of the treatment effect, as the striatum prepares behavioral plans under the direction of prefrontal and premotor systems (Kermadi and Joseph, 1995).
Because of the key role of frontostriatal systems in procedural learning, results from oculomotor studies of Parkinson's disease (PD) are relevant to our findings. PD has an established impact on cognitive abilities subserved by frontostriatal circuitry, including procedural learning and working memory (Hodgson et al, 1999). Investigations of internally generated saccades in patients with PD using predictive and memory-guided saccade tasks have reported reduced accuracy of responses (Chan et al, 2005; Crawford et al, 1989; Kimmig et al, 2002). Hence, the dopaminergic effects of risperidone may in some ways be analogous to the effect of PD on the dopamine systems in striatum, with its consequent adverse effect on thalamocortical drive and therefore the ability of premotor cortex to initiate behavior without sensory guidance (Grafton, 2004).
It is important to highlight that treatment-related change in predictive saccades was not a simple problem of motor control, as there was no abnormality in sensory-guided responses. Also, EPS ratings were low after treatment (Table 1), and the change in predictive saccade gain was not related to changes in manual motor abilities on neuropsychological tests that require sensory-guided responses as do sensory-guided saccades. The reductions in predictive saccade accuracy, in the context of minimal changes in sensory-guided saccades, manual visuomotor control, or EPS, suggest that the predictive saccade task may provide an especially sensitive biomarker of D2 blockade on frontostriatal systems.
Spatial Working Memory
Because performance of the predictive saccade task requires the maintenance and retrieval of spatiotemporal information to guide behavior, spatial working memory is important in facilitating procedural learning of spatially guided motor sequences. Patients with focal prefrontal lesions have working memory deficits and also procedural learning deficits on serial reaction time tasks (Gomez et al, 1999), and spatial working memory deficits are related to reduced saccade gain on the predictive saccade task in schizophrenia (Hutton et al, 2001).
The impact of antipsychotics on working memory systems remains somewhat ambiguous. Though some studies report a beneficial effect (McGurk et al, 2005), we previously reported in two independent samples a worsening of spatial working memory on an oculomotor-delayed response (ODR) task after risperidone treatment (Reilly et al, 2006, 2007). The ODR task places heavier demands on maintenance rather than manipulation aspects of working memory compared to most neuropsychological tests, and therefore it is more similar in cognitive demand to the requirements of the predictive saccade task.
Working memory relies on the activation of prefrontal cortical D1 receptors in PFC (Goldman-Rakic, 1999; Lidow et al, 1997). Destruction of dopamine terminals in the PFC disrupts the integrity of short-term working memory (Seamans et al, 1998), and direct injections of D1 antagonists into the dorsolateral PFC disrupt memory-guided but not sensory-guided saccades (Sawaguchi and Goldman-Rakic, 1994). Given that antipsychotic medication induces robust downregulation of prefrontal D1 receptors (Lidow et al, 1997) and, in turn, working memory impairments that can be reversed by short-term treatment with a D1 agonist (Castner et al, 2000), it is conceivable that reduced fidelity of internal spatial representations could be, at least in part, a cause of decreased predictive saccade accuracy after risperidone treatment. One caveat is that Lidow et al (1997) demonstrated D1 changes in monkeys after 6 months of antipsychotic drug administration, and whether they are present to a neurophysiologically significant level in humans after 6 weeks of treatment is not known.
Spatial Mapping and Memory
Several lines of evidence point to abnormal function and anatomy of the hippocampus in schizophrenia (Heckers, 2001). Wilkerson and Levin (1999) demonstrated that infusion of a D2 antagonist into the hippocampus significantly impaired spatial working memory in rats. Another animal study documented decreased local cerebral glucose utilization in the hippocampus following acute administration of risperidone (Huang et al, 1999). These findings suggest that the hippocampus is affected by risperidone, possibly in ways that could alter the ability of the hippocampus to encode spatial location information necessary for successful performance of the predictive saccade task (Simo et al, 2005).
CONCLUSION
Although there were no pretreatment abnormalities in procedural learning in antipsychotic-naive schizophrenia patients, reductions in the accuracy of predictive saccades were observed after risperidone treatment. The most parsimonious cause of this treatment effect is an alteration of dopamine regulation in striatum, which could reduce thalamocortical facilitation of premotor systems and thus lower the amplitude of voluntary motor actions initiated without sensory guidance. Effects of risperidone on spatial working memory or spatial mapping systems might also contribute to the observed treatment-related neurobehavioral changes.
Because there was no reduction in the accuracy of sensory-guided saccades after treatment, the deficit in predictive saccades was not due to a simple motor system disturbance. Rather, the problem was more cognitive in nature, involving the initiation of accurate responses based on internal spatial representations and behavioral plans. The observation of such an impairment, which is consistent with animal models of the effects of dopamine receptor blockade (Wang et al, 2004) and observations in PD (Hodgson et al, 1999), stands out by way of comparison with the pattern of generalized enhancement on neuropsychological tests reported in clinical trials with risperidone and other antipsychotic treatments (Harvey et al, 2005; Keefe et al, 2006; Woodward et al, 2005). Findings from the present study, and others (Reilly et al, 2006; Sweeney et al, 1997), suggest that translational oculomotor biomarkers may provide sensitive and specific tools for drug discovery and evaluation by parsing drug effects on discrete neurocognitive operations.
References
Ammons CH, Ammons RB (1962). The Quick Test (QT): provisional manual. Psychol Rep 11: 111–116.
Andreasen NC (1984a). Scale for the Assessment of Positive Symptoms. University of Iowa: Iowa City.
Andreasen NC (1984b). Scale for the Assessment of Negative Symptoms. University of Iowa: Iowa City.
Becker W (1989). Metrics. In: Wurtz RH, Goldberg ME (eds). The Neurobiology of Saccadic Eye Movements. Elsevier: Amsterdam. pp 13–61.
Bedard MA, Scherer H, Stip E, Cohen H, Rodriguez JP, Richer F (2000). Procedural learning in schizophrenia: further consideration on the deleterious effect of neuroleptics. Brain Cogn 43: 31–39.
Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA et al (2000). Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry 157: 549–559.
Bleuler E (1952). Dementia Praecox or the Group of Schizophrenia. International Universities Press: New York.
Castner SA, Williams GV, Goldman-Rakic PS (2000). Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science 287: 2020–2022.
Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP (2005). Deficits in saccadic eye-movement control in Parkinson's disease. Neuropsychologia 43: 784–796.
Clementz BA, McDowell JE, Zisook S (1994). Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol 103: 277–287.
Cohen NJ, Eichenbaum H, Deacedo BS, Corkin S (1985). Different memory systems underlying acquisition of procedural and declarative knowledge. Ann NY Acad Sci 444: 54–71.
Crawford TJ, Henderson L, Kennard C (1989). Abnormalities of non-visually guided eye movements in Parkinson's disease. Brain 112: 1573–1586.
Crawford TJ, Haeger B, Kennard C, Henderson L (1995a). Saccadic abnormalities in psychotic patients. II. The role of neuroleptic treatment. Psychol Med 25: 473–483.
Crawford TJ, Haeger B, Kennard C, Reveley MA, Henderson L (1995b). Saccadic abnormalities in psychotic patients. I. Neuroletpic-free psychotic patients. Psychol Med 25: 461–471.
Everling S, Munoz DP (2000). Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci 20: 387–400.
First M, Spitzer RL, Gibbon M, Williams JBW (1995). Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P). New York State Psychiatric Institute: New York.
Fischer B, Weber H, Biscaldi M, Aiple F, Otto P, Stuhr V (1993). Separate populations of visually guided saccades in humans: reaction times and amplitudes. Exp Brain Res 92: 528–541.
Garraux G, McKinney C, Wu T, Kansaku K, Nolte G, Hallett M (2005). Shared brain areas but not functional connections controlling movement timing and order. J Neurosci 25: 5290–5297.
Goldman-Rakic PS (1999). The relevance of the dopamine D1 receptor in the cognitive symptoms of schizophrenia. Neuropsychopharmacology 21: S6.
Gomez BM, Grafman J, Pascual-Leone A, Garcia-Monco JC (1999). Procedural learning is impaired in patients with prefrontal lesions. Neurology 52: 1853–1860.
Grafton ST (2004). Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol 14: 715–719.
Green MF, Kern RS, Williams O, McGurk S, Kee K (1997). Procedural learning in schizophrenia: evidence from serial reaction time. Cogn Neuropsychiatry 2: 123–134.
Guitton D, Buchtel HA, Douglas RM (1985). Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res 58: 455–472.
Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62.
Harris MSH, Reilly JL, Keshavan MS, Sweeney JA (2006). Longitudinal studies of antisaccades in first-episode schizophrenia. Psychol Med 36: 485–494.
Harvey PD, Rabinowitz J, Eerdekens M, Davidson M (2005). Treatment of cognitive impairment in early psychosis: a comparison of risperidone and haloperidol in a large long-term trial. Am J Psychiatry 162: 1888–1895.
Heckers S (2001). Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 11: 520–528.
Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K et al (1999). Parallel neural networks for learning sequential procedures. Trends Neurosci 22: 464–471.
Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA (2004). Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naive patients with schizophrenia. Schizophr Res 68: 49–63.
Hinton SC, Meck WH (1997). The ‘internal clocks’ of circadian and interval timing. Endeavour 21: 82–87.
Hodgson TL, Dittrich WH, Henderson L, Kennard C (1999). Eye movements and spatial working memory in Parkinson's disease. Neuropsychologia 37: 927–938.
Hommer DW, Clem T, Litman R, Pickar D (1991). Maladaptive anticipatory saccades in schizophrenia. Biol Psychiatry 30: 779–794.
Huang Y, Tsai S, Huang J, Sim C (1999). The effect of acute administration of risperidone on local cerebral glucose utilization in the rate. Eur J Pharmacol 370: 257–261.
Huston P, Shakow D (1948). Learning in schizophrenia I. Pursuit learning. J Pers 17: 52–74.
Hutton SB, Cuthbert I, Crawford TJ, Kennard C, Barnes TRE, Joyce EM (2001). Saccadic hypometria in drug-naïve and drug treated schizophrenia patients: a working memory deficit? Psychophysiology 38: 125–132.
Keefe RS, Young CA, Rock SL, Purdon SE, Gold JM, Breier A (2006). One-year double-blind study of the neurocognitive efficacy of olanzapine, risperidone, and haloperidol in schizophrenia. Schizophr Res 81: 1–15.
Kermadi I, Joseph JP (1995). Activity in the caudate nucleus of monkey during spatial sequencing. J Neurophysiol 74: 911–933.
Kern RS, Green MF, Marshall BD, Wirshing WC, Wirshing D, McGurk S et al (1998). Risperidone vs haloperidol on reaction time, manual dexterity, and motor learning in treatment-resistant schizophrenia patients. Biol Psychiatry 44: 726–732.
Kimmig H, Haussmann K, Mergner T, Lucking CH (2002). What is pathological with gaze shift fragmentation in Parkinson's disease? J Neurol 249: 683–692.
Kraepelin E (1925). Dementia Praecox and Paraphrenia. Livingston: Edinburgh.
Krebs HI, Brashers-Krug T, Rauch SL, Savage CR, Hogan N, Rubin RH et al (1998). Robot-aided functional imaging: application to a motor learning study. Hum Brain Mapp 6: 59–72.
Krebs MO, Gut-Fayand A, Amado I, Daban C, Bourdel MC, Poirier MF et al (2001). Impairment of predictive saccades in schizophrenia. Neuroreport 12: 465–469.
Kumari V, Corr PJ, Mulligan OF, Cotter PA, Checkley SA, Gray JA (1997). Effects of acute administration of d-amphetamine and haloperidol on procedural learning in man. Psychopharmacology 129: 271–276.
Kumari V, Gray JA, Honey GD, Soni W, Bullmore ET, Williams SC et al (2002). Procedural learning in schizophrenia: a functional magnetic resonance imaging investigation. Schizophr Res 57: 97–107.
Lencer R, Malchow CP, Trillenberg-Krecker K, Schwinger E, Arolt V (2000). Eye-tracking dysfunction (ETD) in families with sporadic and familial schizophrenia. Biol Psychiatry 47: 391–401.
Lidow MS, Elsworth JD, Goldman-Rakic PS (1997). Down-regulation of the D1 and D5 dopamine receptors in the primate prefrontal cortex by chronic treatment with antipsychotic drugs. J Pharmacol Exp Ther 281: 597–603.
McDowell JE, Clementz BA, Wixted JT (1996). Timing and amplitude of saccades during predictive tracking in schizophrenia. Psychophysiology 33: 93–101.
McEvoy JP, Hogarty GE, Steingard S (1991). Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psychiatry 48: 739–745.
McGurk SR, Carter C, Goldman R, Green MF, Marder SR, Xie H et al (2005). The effects of clozapine and risperidone on spatial working memory in schizophrenia. Am J Psychiatry 162: 1013–1016.
Overall JE, Gorham DR (1962). The brief psychiatric rating scale. Psychol Rep 10: 799–812.
Pierrot-Deseilligny C (1994). Saccade and smooth-pursuit impairment after cerebral hemispheric lesions. Eur Neurol 34: 121–134.
Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY et al (2005). The neural correlates of motor skill automaticity. J Neurosci 25: 5356–5364.
Purdon SE, Woodward N, Lindborg SR, Stip E (2003). Procedural learning in schizophrenia after 6 months of double blind treatment with olanzapine, risperidone and haloperidol. Psychopharmacology 169: 390–397.
Reilly JL, Harris MSH, Keshavan MS, Sweeney JA (2006). Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiatry 63: 1189–1197.
Reilly JL, Harris MSH, Khine TT, Keshavan MS, Sweeney JA (2007). Antipsychotic drugs exacerbate impairment on a working memory task in first-episode schizophrenia. Biol Psychiatry 62: 818–821.
Ross DE, Thaker GK, Buchanan RW, Kirkpatrick B, Lahti AC, Medoff D et al (1997). Eye tracking disorder in schizophrenia is characterized by specific ocular motor defects and is associated with the deficit syndrome. Biol Psychiatry 42: 781–796.
Salmon DP, Butters N (1995). Neurobiology of skill and habit learning. Curr Opin Neurobiol 5: 184–190.
Sawaguchi T, Goldman-Rakic PS (1994). The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol 71: 515–528.
Seamans JK, Floresco SB, Phillips AG (1998). D1 receptor modulation of hippocampal–prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci 18: 1613–1621.
Simo LS, Krisky CM, Sweeney JA (2005). Functional neuroanatomy of anticipatory behavior: dissociation between sensory-driven and memory-driven systems. Cereb Cortex 15: 1982–1991.
Sweeney JA, Bauer KS, Keshavan MS, Haas GL, Schooler NR, Kroboth PD (1997). Adverse effects of risperidone on eye movement activity: a comparison of risperidone and haloperidol in antipsychotic-naive schizophrenic patients. Neuropsychopharmacology 16: 217–228.
Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL et al (1998). Eye tracking abnormalities in schizophrenia: evidence for dysfunction in the frontal eye fields. Biol Psychiatry 44: 698–708.
Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR et al (1996). Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol 75: 454–468.
Thaker GK, Ross DE, Buchanan RW, Moran MJ, Lahti A, Kim C et al (1996). Does pursuit abnormality in schizophrenia represent a deficit in the predictive mechanism? Psychiatry Res 59: 221–237.
Wang M, Vijayraghavan S, Goldman-Rakic PS (2004). Selective D2 receptor actions on the functional circuitry of working memory. Science 303: 853–856.
Wenban-Smith MG, Findlay JM (1991). Express saccades: is there a separate population in humans? Exp Brain Res 87: 218–222.
Wilkerson A, Levin ED (1999). Ventral hippocampal dopamine D1 and D2 systems and spatial working memory in rats. Neuroscience 89: 743–749.
Woodward ND, Purdon SE, Meltzer HY, Zald DH (2005). A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol 8: 457–472.
Zedkova L, Woodward ND, Harding I, Tibbo PG, Purdon SE (2007). Procedural learning in schizophrenia investigated with functional magnetic resonance imaging. Schizophr Res 88: 198–207.
Acknowledgements
We thank Dr Gretchen Haas, Dr Cameron Carter, Dr Debra Montrose, and the clinical core staff of the Center for the Neuroscience of Mental Disorders (Dr David Lewis, director) for their assistance in diagnostic and psychopathological assessments.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICTS OF INTEREST
This publication was supported by funds received from the National Institute of Health (NIH) grants MH62134, MH45156 and MH01433, the NIH/NCRR/GCRC grant M01 RR00056, and Janssen grant RIS-INT-35.
The authors declare that over the past three years JAS has received compensation from Abbott Laboratories, Eli Lilly and Company, Janssen, and Roche. MSK has received compensation from Janssen, Pfizer, Bristol-Myers Squibb, Sanofi and Astra Zeneca. The other authors have no conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Harris, M., Wiseman, C., Reilly, J. et al. Effects of Risperidone on Procedural Learning in Antipsychotic-Naive First-Episode Schizophrenia. Neuropsychopharmacol 34, 468–476 (2009). https://doi.org/10.1038/npp.2008.79
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2008.79
Keywords
This article is cited by
-
Pharmacokinetic patterns of risperidone-associated adverse drug reactions
European Journal of Clinical Pharmacology (2016)
-
Probabilistic classification and gambling in patients with schizophrenia receiving medication: comparison of risperidone, olanzapine, clozapine and typical antipsychotics
Psychopharmacology (2012)