Abstract
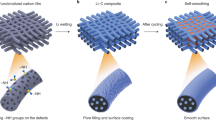
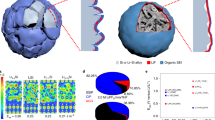
For future applications in portable electronics, electric vehicles and grid storage, batteries with higher energy storage density than existing lithium ion batteries need to be developed. Recent efforts in this direction have focused on high-capacity electrode materials such as lithium metal, silicon and tin as anodes, and sulphur and oxygen as cathodes. Lithium metal would be the optimal choice as an anode material, because it has the highest specific capacity (3,860 mAh g–1) and the lowest anode potential of all. However, the lithium anode forms dendritic and mossy metal deposits, leading to serious safety concerns and low Coulombic efficiency during charge/discharge cycles. Although advanced characterization techniques have helped shed light on the lithium growth process, effective strategies to improve lithium metal anode cycling remain elusive. Here, we show that coating the lithium metal anode with a monolayer of interconnected amorphous hollow carbon nanospheres helps isolate the lithium metal depositions and facilitates the formation of a stable solid electrolyte interphase. We show that lithium dendrites do not form up to a practical current density of 1 mA cm–2. The Coulombic efficiency improves to ∼99% for more than 150 cycles. This is significantly better than the bare unmodified samples, which usually show rapid Coulombic efficiency decay in fewer than 100 cycles. Our results indicate that nanoscale interfacial engineering could be a promising strategy to tackle the intrinsic problems of lithium metal anodes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Whittingham, M. S. Electrical energy storage and intercalation chemistry. Science 192, 1126–1127 (1976).
Ohzuku, T., Iwakoshi, Y. & Sawai, K. Formation of lithium–graphite intercalation compounds in nonaqueous electrolytes and their application as a negative electrode for a lithium ion (shuttlecock) cell. J. Electrochem. Soc. 140, 2490–2498 (1993).
Chan, C. K. et al. High-performance lithium battery anodes using silicon nanowires. Nature Nanotech. 3, 31–35 (2008).
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J. M. Li–O2 and Li–S batteries with high energy storage. Nature Mater. 11, 19–29 (2012).
Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—the solid electrolyte interphase model. J. Electrochem. Soc. 126, 2047–2051 (1979).
Aurbach, D. et al. Attempts to improve the behavior of Li electrodes in rechargeable lithium batteries J. Electrochem. Soc. 150, L6 (2003).
Ota, H. et al. Characterization of lithium electrode in lithium imides/ethylene carbonate and cyclic ether electrolytes: II. Surface chemistry. J. Electrochem. Soc. 151, A437–A446 (2004).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004).
Bhattacharyya, R. et al. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nature Mater. 9, 504–510 (2010).
Chandrashekar, S. et al. 7Li MRI of Li batteries reveals location of microstructural lithium. Nature Mater. 11, 311–315 (2012).
Harry, K. J. et al. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nature Mater. 13, 69–73 (2013).
Von Sacken, U., Nodwell, E., Sundher, A. & Dahn, J. R. Comparative thermal stability of carbon intercalation anodes and lithium metal anodes for rechargeable lithium batteries. J. Power Sources 54, 240–245 (1995).
Chazalviel, J. N. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 42, 7355–7367 (1990).
Rosso, M. et al. Onset of dendritic growth in lithium/polymer cells. J. Power Sources 97-98, 804–806 (2001).
Yu, X., Bates, J. B., Jellison, G. E. & Hart, F. X. A stable thin-film lithium electrolyte: lithium phosphorus oxynitride. J. Electrochem. Soc. 144, 524–532 (1997).
Nimon, Y. S., Chu, M-Y. & Visco, S. J. Coated lithium electrodes. US patent US6537701 B1 (2003).
Kamaya, N. et al. A lithium superionic conductor. Nature Mater. 10, 682–686 (2011).
Stone, G. M. et al. Resolution of the modulus versus adhesion dilemma in solid polymer electrolytes for rechargeable lithium metal batteries. J. Electrochem. Soc. 159, A222–A227 (2012).
Croce, F., Persi, L., Ronci, F. & Scrosati, B. Nanocomposite polymer electrolytes and their impact on the lithium battery technology. Solid State Ionics 135, 47–52 (2000).
Zaghib, K. Lithium metal vs. Li-ion batteries: challenges and opportunities. ECS Meeting Abstracts MA2013-02, 952 (2013).
Murugan, R., Thangadurai, V. & Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12 . Angew. Chem. Int. Ed. 46, 7778–7781 (2007).
Thangadurai, V., Narayanan, S. & Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: critical review. Chem. Soc. Rev. 43, 4714–4727 (2014).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Kim, K. H. et al. Characterization of the interface between LiCoO2 and Li7La3Zr2O12 in an all-solid-state rechargeable lithium battery. J. Power Sources 196, 764–767 (2011).
Crowther, O. & West, A. C. Effect of electrolyte composition on lithium dendrite growth. J. Electrochem. Soc. 155, A806–A811 (2008).
Hirai, T., Yoshimatsu, I. & Yamaki, J-I. Effect of additives on lithium cycling efficiency. J. Electrochem. Soc. 141, 2300–2305 (1994).
Ding, F. et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013).
Marchioni, F. et al. Protection of lithium metal surfaces using chlorosilanes. Langmuir 23, 11597–11602 (2007).
Ishikawa, M., Kawasaki, H., Yoshimoto, N. & Morita, M. Pretreatment of Li metal anode with electrolyte additive for enhancing Li cycleability. J. Power Sources 146, 199–203 (2005).
Aurbach, D., Zinigrad, E., Cohen, Y. & Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ionics 148, 405–416 (2002).
Gireaud, L. et al. Lithium metal stripping/plating mechanisms studies: a metallurgical approach. Electrochem. Commun. 8, 1639–1649 (2006).
Suk, J. W., Murali, S., An, J. & Ruoff, R. S. Mechanical measurements of ultra-thin amorphous carbon membranes using scanning atomic force microscopy. Carbon 50, 2220–2225 (2012).
Xia, Y., Gates, B., Yin, Y. & Lu, Y. Monodispersed colloidal spheres: old materials with new applications. Adv. Mater. 12, 693–713 (2000).
Larson, D. M., Downing, K. H. & Glaeser, R. M. The surface of evaporated carbon films is an insulating, high-bandgap material. J. Struct. Biol. 174, 420–423 (2011).
Blue, M. D. & Danielson, G. C. Electrical properties of arc-evaporated carbon films. J. Appl. Phys. 28, 583–586 (1957).
Arie, A. A. & Lee, J. K. Electrochemical characteristics of lithium metal anodes with diamond like carbon film coating layer. Diamond Relat. Mater. 20, 403–408 (2011).
Huang, J. Y. et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode. Science 330, 1515–1520 (2010).
McDowell, M. T. et al. In situ TEM of two-phase lithiation of amorphous silicon nanospheres. Nano Lett. 13, 758–764 (2013).
Aurbach, D., Zinigrad, E., Teller, H. & Dan, P. Factors which limit the cycle life of rechargeable lithium (metal) batteries. J. Electrochem. Soc. 147, 1274–1279 (2000).
Aurbach, D., Gofer, Y. & Langzam, J. The correlation between surface chemistry, surface morphology, and cycling efficiency of lithium electrodes in a few polar aprotic systems. J. Electrochem. Soc. 136, 3198–3205 (1989).
Gofer, Y., Ben-Zion, M. & Aurbach, D. Solutions of LiAsF6 in 1,3-dioxolane for secondary lithium batteries. J. Power Sources 39, 163–178 (1992).
Koch, V. R., Goldman, J. L., Mattos, C. J. & Mulvaney, M. Specular lithium deposits from lithium hexafluoroarsenate/diethyl ether electrolytes. J. Electrochem. Soc. 129, 1–4 (1982).
Shimmin, R. G., DiMauro, A. J. & Braun, P. V. Slow vertical deposition of colloidal crystals: a Langmuir–Blodgett process? Langmuir 22, 6507–6513 (2006).
Acknowledgements
G.Z. acknowledges financial support from Agency for Science, Technology and Research (A*STAR), Singapore. The authors thank A. Jaffe for help with the Fourier transform infrared measurement and H. Yuan for help with the conductivity measurements. H.L. was supported by the Basic Science Research Program through the National Research Foundation of Korea (contract no. NRF-2012R1A6A3A03038593).
Author information
Authors and Affiliations
Contributions
G.Z and Y.C. conceived and designed the experiments. G.Z performed the experiments. S.W.L. performed the numerical simulation and provided data analysis. H.W.L. conducted in situ TEM characterization. G.Z. and Y.C. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 47092 kb)
Supplementary Movie
Supplementary Movie (MOV 1604 kb)
Rights and permissions
About this article
Cite this article
Zheng, G., Lee, S., Liang, Z. et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nature Nanotech 9, 618–623 (2014). https://doi.org/10.1038/nnano.2014.152
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2014.152
This article is cited by
-
Engineering Strategies for Suppressing the Shuttle Effect in Lithium–Sulfur Batteries
Nano-Micro Letters (2024)
-
Lithium-induced graphene layer containing Li3P alloy phase to achieve ultra-stable electrode interface for lithium metal anode
Rare Metals (2024)
-
From Liquid to Solid-State Lithium Metal Batteries: Fundamental Issues and Recent Developments
Nano-Micro Letters (2024)
-
Design Strategies for Aqueous Zinc Metal Batteries with High Zinc Utilization: From Metal Anodes to Anode-Free Structures
Nano-Micro Letters (2024)
-
A corrosion inhibiting layer to tackle the irreversible lithium loss in lithium metal batteries
Nature Communications (2023)