Abstract
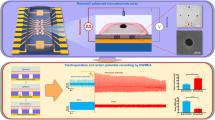
Recording intracellular (IC) bioelectrical signals is central to understanding the fundamental behaviour of cells and cell networks in, for example, neural and cardiac systems1,2,3,4. The standard tool for IC recording, the patch-clamp micropipette5 is applied widely, yet remains limited in terms of reducing the tip size, the ability to reuse the pipette5 and ion exchange with the cytoplasm6. Recent efforts have been directed towards developing new chip-based tools1,2,3,4,7,8,9,10,11,12,13, including micro-to-nanoscale metal pillars7,8,9, transistor-based kinked nanowires10,11 and nanotube devices12,13. These nanoscale tools are interesting with respect to chip-based multiplexing, but, so far, preclude targeted recording from specific cell regions and/or subcellular structures. Here we overcome this limitation in a general manner by fabricating free-standing probes in which a kinked silicon nanowire with an encoded field-effect transistor detector serves as the tip end. These probes can be manipulated in three dimensions within a standard microscope to target specific cells or cell regions, and record stable full-amplitude IC action potentials from different targeted cells without the need to clean or change the tip. Simultaneous measurements from the same cell made with free-standing nanowire and patch-clamp probes show that the same action potential amplitude and temporal properties are recorded without corrections to the raw nanowire signal. In addition, we demonstrate real-time monitoring of changes in the action potential as different ion-channel blockers are applied to cells, and multiplexed recording from cells by independent manipulation of two free-standing nanowire probes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Parpura, V. Bionanoelectronics: getting close to the action. Nature Nanotech. 7, 143–145 (2012).
Spira, M. & Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nature Nanotech. 8, 83–94 (2013).
Duan, X., Fu, T-M., Liu, J. & Lieber, C. M. Nanoelectronics–biology frontier: from nanoscopic probes for action potential recording in live cells to three-dimensional cyborg tissues. Nano Today 8, 351–373 (2013).
Dunlop, J., Bowlby, M., Peri, R., Vasilyev, D. & Arias, R. High-throughput electrophysiology: an emerging paradigm for ion-channel screening and physiology. Nature Rev. Drug Discov. 7, 358–368 (2008).
Molleman, A. Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology (Wiley, 2003).
Sakmann B. & Neher E. Single-Channel Recording (Plenum Press, 1995).
Xie, C., Lin, Z., Hanson, L., Cui, Y. & Cui, B. Intracellular recording of action potentials by nanopillar electroporation. Nature Nanotech. 7, 185–190 (2012).
Robinson, J. T. et al. Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits. Nature Nanotech. 7, 180–184 (2012).
Hai, A., Shappir, J. & Spira, M. E. Long-term, multisite, parallel, in-cell recording and stimulation by an array of extracellular microelectrodes. J. Neurophysiol. 104, 559–568 (2010).
Tian, B. et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329, 830–834 (2010).
Jiang, Z., Qing, Q., Xie, P., Gao, R. X. & Lieber, C. M. Kinked p–n junction nanowire probes for high spatial resolution sensing and intracellular recording. Nano Lett. 12, 1711–1716 (2012).
Duan, X. J. et al. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nature Nanotech. 7, 174–179 (2012).
Gao, R. X. et al. Outside looking in: nanotube transistor intracellular sensors. Nano Lett. 12, 3329–3333 (2012).
Tian, B. et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nature Mater. 11, 986–994 (2012).
Tian, B., Xie, P., Kempa, T. J., Bell, D. C. & Lieber, C. M. Single-crystalline kinked semiconductor nanowire superstructures. Nature Nanotech. 4, 824–829 (2009).
Bers, D. M. Cardiac excitation–contraction coupling. Nature 415, 198–205 (2002).
Zipes, D. P. & Jalife, J. Cardiac Electrophysiology: From Cell to Bedside 2nd edn (Saunders, 2009).
Chernomordik, L. V. & Kozlov, M. M. Mechanics of membrane fusion. Nature Struct. Mol. Biol. 15, 675–683 (2008).
Nicolas, J., Mura, S., Brambilla, D., Mackiewicz, N. & Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 42, 1147–1235 (2013).
Subbiah, R., Veerapandian, M., & Yun, K. S. Nanoparticles: functionalization and multifunctional applications in biomedical sciences. Curr. Med. Chem. 17, 4559–4577 (2010).
Kotov, N. A. et al. Nanomaterials for neural interfaces. Adv. Mater. 21, 3970–4004 (2009).
Hai, A., Shappir, J. & Spira, M. E. In-cell recordings by extracellular microelectrodes. Nature Methods 7, 200–202 (2010).
Szentandrassy, N. et al. Powerful technique to test selectivity of agents acting on cardiac ion channels: the action potential voltage-clamp. Curr. Med. Chem. 18, 3737–3756 (2011).
Viatchenko-Karpinski, S. et al. Intracellular Ca2+ oscillations drive spontaneous contractions in cardiomyocytes during early development. Proc. Natl Acad. Sci. USA 96, 8259–8264 (1999).
Davie, J. T. et al. Dendritic patch-clamp recording. Nature Protocols 1, 1235–1247 (2006).
Xu, L. et al. Design and synthesis of diverse functional kinked nanowire structures for nanoelectronic bioprobes. Nano Lett. 13, 746–751 (2013).
Akbarali, H. I., Wyse, D. G. & Giles, W. R. Ionic currents in single cells from human cystic artery. Circ. Res. 70, 536–545 (1992).
Chilton, L. et al. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am. J. Physiol. Heart Circ. Physiol. 288, H2931–H2939 (2005).
Clark, B. A. & Mobbs, P. Voltage-gated currents in rabbit retinal astrocytes. Eur. J. Neurosci. 6, 1406–1414 (1994).
Acknowledgements
C.M.L. acknowledges support of this work by a National Institutes of Health Director's Pioneer Award (5DP1OD003900), National Basic Research Program of China (2013CB934103), and International Science & Technology Corporation Program of China (2013DFA50840).
Author information
Authors and Affiliations
Contributions
Q.Q., Z.J. and C.M.L. designed the experiments, Q.Q., Z.J., and L.X. performed the experiments, R.G. helped in cardiomyocyte culture experiments, Q.Q., Z.J., L.X. and C.M.L. analysed data and Q.Q., Z.J. and C.M.L. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 670 kb)
Rights and permissions
About this article
Cite this article
Qing, Q., Jiang, Z., Xu, L. et al. Free-standing kinked nanowire transistor probes for targeted intracellular recording in three dimensions. Nature Nanotech 9, 142–147 (2014). https://doi.org/10.1038/nnano.2013.273
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2013.273
This article is cited by
-
Review of Neural Interfaces: Means for Establishing Brain–Machine Communication
SN Computer Science (2023)
-
Biointerface design for vertical nanoprobes
Nature Reviews Materials (2022)
-
Towards Green 3D-Microfabrication of Bio-MEMS Devices Using ADEX Dry Film Photoresists
International Journal of Precision Engineering and Manufacturing-Green Technology (2022)
-
Semi-Implantable Bioelectronics
Nano-Micro Letters (2022)
-
Three-dimensional transistor arrays for intra- and inter-cellular recording
Nature Nanotechnology (2022)