Abstract
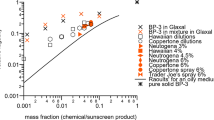
Numerous reports have described the superior properties of nanoparticles and their diverse range of applications. Issues of toxicity1,2, workplace safety3,4 and environmental impact5,6,7,8,9 have also been a concern. Here we show a theoretical comparison of how the size of titanium dioxide nanoparticles and their concentration in sunscreens can affect efficacy, aesthetics and potential toxicity from free radical production. The simulation results reveal that, unless very small nanoparticles can be shown to be safe, there is no combination of particle size and concentration that will deliver optimal performance in terms of sun protection and aesthetics. Such a theoretical method complements well the experimental approach for identifying these characteristics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hoet, P. H. M., Brüske-Hohlfeld, I. & Salata, O. V. Nanoparticles—known and unknown health risks. J. Nanobiotech. 22, 12 (2004).
Magrez, A. et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 6, 1121–1125 (2006).
Donaldson, K. et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 92, 5–22 (2006).
Bartis, J. & Landree, E. Nanomaterials in the Workplace: Policy and Planning Workshop on Occupational Safety and Health (RAND, 2006).
Donaldson, K., Stone, V., Tran, C. L., Kreyling, W. & Borm, P. J. A. Nanotoxicology. Occup. Environ. Med. 61, 727–728 (2004).
Oberdörster, G., Oberdörster, E. & Oberdörster, J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 113, 823–839 (2005).
Barnard, A. S. Nanohazards: knowledge is our first defence. Nature Mater. 5, 245–248 (2006).
Baun, A., Hartmann, N., Grieger, K., & Kusk, K. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology 17, 387–395 (2008).
Nel, A., Xia, T., Mädler, L. & Li, N. Toxic potential of materials at the nanolevel. Science 311, 622–627 (2006).
Tyner, K. M. et al. Comparing methods for detecting and characterizing metal oxide nanoparticles in unmodified commercial sunscreens. Nanomedicine 4, 145–159 (2009).
Hirakawa, T., Yawata, K. & Nosaka, Y. Photocatalytic reactivity for O2•− and OH• radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A 325, 105–111 (2007).
Wiseman, H. & Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem. J. 313, 17–29 (1996).
Serpone, N., Salinaro, A. & Emeline, A. Deleterious effects of sunscreen titanium dioxide nanoparticles on DNA: efforts to limit DNA damage by particle surface modification. Proc. SPIE 4258, 86–98 (2001).
Kertesz, Zs., Szikszai, Z. & Kiss, A. Z. Quality of skin as a barrier to ultra-fine particles. Contribution of the IBA Group to the NANODERM EU-5 Project (2003–2004).
Vogt, A. et al. 40 nm, but not 750 or 1500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J. Invest. Dermatol. 126, 1316–1322 (2006).
Zhang, L. W. & Monteiro-Riviere, N. A. Assessment of quantum dot penetration into intact, tape-stripped, abraded and flexed rat skin. Skin Pharmacol. Physiol. 21, 166–180 (2008).
Barker, P. J. & Branch, A. The interaction of modern sunscreen formulations with surface coatings. Prog. Org. Coatings 62, 313–320 (2008).
Barnard, A. S. How can ab initio simulations address risks in nanotech? Nature Nanotech. 4, 332–335 (2009).
Villalobos-Hernández, J. R. & Müller-Goymann, C. C. Sun protection enhancement of titanium dioxide crystals by the use of carnauba wax nanoparticles: the synergistic interaction between organic and inorganic sunscreens at nanoscale. Int. J. Pharmacol. 322, 161–170 (2006).
Barnard, A. S. & Xu, H. An environmentally sensitive phase map of titania nanocrystals. ACS Nano 2, 2237–2242 (2008).
Yang, H. G. et al. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 453, 638–641 (2008).
Ohno, T., Sarukawa, K. & Matsumura, M. Crystal faces of rutile and anatase TiO2 particles and their roles in photocatalytic reactions. New J. Chem. 26, 1167–1170 (2002).
Jiang, J. et al. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicol. 2, 33–42 (2008).
Mckinlay, A. F. & Diffey, B. L. A reference action spectrum of ultraviolet induced erythema in human skin, in Human Exposure to Ultraviolet Radiation: Risks and Regulations 83–87 (Elsevier, 1987).
Mie, G. Beiträge zur optik trüber medien, speziell kolloidaler metallösungen. Ann. Phys. 330, 377–445 (1908).
Thiele, E. S. & French, R. H. Light-scattering properties of representative, morphological rutile titania particles using a finite-element method. J. Am. Ceram. Soc. 81, 469–479 (1998).
Popov, A. P., Priezzhev, A. V., Lademann, J. & Myllylä, R. TiO2 nanoparticles as an effective UV-B radiation skin-protective compound in sunscreens. J. Phys. D 38, 2564–2570 (2005).
Tuchin, V. V. Tissue Optics (SPIE Optical Engineering Press, 2000).
Barnard, A. S. A thermodynamic model for the shape and stability of twinned nanostructures. J. Phys. Chem. B 110, 24498–24504 (2006).
Barnard, A. S. & Curtiss, L. A. Prediction of TiO2 nanoparticle phase and shape transitions controlled by surface chemistry. Nano Lett. 5, 1261–1266 (2005).
Acknowledgements
A.S.B. acknowledges the support of the Australian Research Council (ARC) (DP0986752), L'Oréal Australia, and the Australian Academy of Sciences, and G. Smith and H. Xu for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 457 kb)
Rights and permissions
About this article
Cite this article
Barnard, A. One-to-one comparison of sunscreen efficacy, aesthetics and potential nanotoxicity. Nature Nanotech 5, 271–274 (2010). https://doi.org/10.1038/nnano.2010.25
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2010.25
This article is cited by
-
In situ electron microscopy techniques for nanoparticle dispersion analysis of commercial sunscreen
Journal of Nanoparticle Research (2023)
-
Fabrication of Metal and Metal Oxide Nanoparticles by Algae and their Toxic Effects
Nanoscale Research Letters (2016)
-
Microglial cells (BV-2) internalize titanium dioxide (TiO2) nanoparticles: toxicity and cellular responses
Environmental Science and Pollution Research (2016)
-
A sunblock based on bioadhesive nanoparticles
Nature Materials (2015)
-
Characterization of physicochemical properties of ivy nanoparticles for cosmetic application
Journal of Nanobiotechnology (2013)