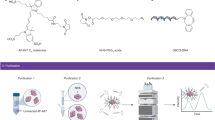
Abstract
An average cell contains thousands of proteins that participate in normal cellular functions, and most diseases are somehow related to the malfunctioning of one or more of these proteins. Protein therapy1, which delivers proteins into the cell to replace the dysfunctional protein, is considered the most direct and safe approach for treating disease. However, the effectiveness of this method has been limited by its low delivery efficiency and poor stability against proteases in the cell, which digest the protein. Here, we show a novel delivery platform based on nanocapsules consisting of a protein core and a thin permeable polymeric shell that can be engineered to either degrade or remain stable at different pHs. Non-degradable capsules show long-term stability, whereas the degradable ones break down their shells, enabling the core protein to be active once inside the cells. Multiple proteins can be delivered to cells with high efficiency while maintaining low toxicity, suggesting potential applications in imaging, therapy and cosmetics fields.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Birch, J. R. & Onakunle, Y. in Therapeutic Proteins, Methods and Protocols 1–16 (Humana Press, 2005).
Wadia, J. S., Becker-Hapak, M. & Dowdy, S. F. Cell-Penetrating Peptides: Processes and Applications 365 (CRC Press, 2002).
Vyas, S. P., Singh, A. & Sihorkar, V. Ligand-receptor-mediated drug delivery: an emerging paradigm in cellular drug targeting. Crit. Rev. Ther. Drug Carrier Syst. 18, 1–76 (2001).
Sato, H., Sugiyama, Y., Tsuji, A. & Horikoshi, I. Importance of receptor-mediated endocytosis in peptide delivery and targeting: kinetic aspects. Adv. Drug Deliv. Rev. 19, 445–467 (1996).
Torchilin, V. P. Recent advances with liposomes as pharmaceutical carriers. Nature Rev. Drug Discov. 4, 145–160 (2005).
Bulmus, V. et al. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J. Control Release 93, 105–120 (2003).
Schwarze, S. R., Ho, A., Vocero-Akbani, A. & Dowdy, S. F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572 (1999).
Heitz, F., Morris, M. C. & Divita, G. Twenty years of cell-penetrating peptides: from molecular mechanism to therapeutics. Br. J. Pharmacol. 157, 195–206 (2009).
Gros, E. et al. A non-covalent peptide-based strategy for protein and peptide nucleic acid delivery. Biochim. Biophys. Acta 1758, 384–393 (2006).
Fagain, C. O. Understanding and increasing protein stability. Biochim. Biophys. Acta 1252, 1–14 (1995).
Hooper, N. M. Proteases in Biology and Medicine (Portland Press, 2002).
Brooks, H., Lebleu, B. & Vives, E. TAT peptide-mediated cellular delivery: back to basics. Adv. Drug Deliv. Rev. 57, 559–577 (2005).
Poon, G. M. K. & Gariepy, J. Cell-surface proteoglycans as molecular portals for cationic peptide and polymer entry into cells. Biochem. Soc. Trans. 35, 778–793 (2007).
Futami, J. et al. Intracellular delivery of proteins into mammalian living cells by polyethylenimine-cationization. J. Biosci. Bioeng. 99, 95–103 (2005).
Fischer, R., Kohler, K., Fotin-Mleczek, M. & Brock, R. A stepwise dissection of the intracellular fate of cationic cell-penetrating peptides. J. Biol. Chem. 279, 12625–12635 (2004).
Akinc, A., Thomas, M., Klibanov, A. M. & Langer, R. Exploring polymethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 7, 657–663 (2004).
Yamauchi, N. et al. Mechanism of synergistic cytotoxic effect between tumor-necrosis-factor and hyperthermia. Jpn J. Canc. Res. 83, 540–545 (1992).
Kaliberov, S. A. et al. Combination of cytosine deaminase suicide gene expression with DR5 antibody treatment increases cancer cell cytotoxicity. Cancer Gene Ther. 13, 203–214 (2006).
Folkes, L. K. & Wardman, P. Oxidative activation of indole-3-acetic acids to cytotoxic species — a potential new role for plant auxins in cancer therapy. Biochem. Pharmacol. 61, 129–136 (2001).
de Melo, M. P., de Lima, T. M., Pithon-Curi, T. C. & Curi, R. The mechanism of indole acetic acid cytotoxicity. Toxicol. Lett. 148, 103–111 (2004).
Fridovich, I. Superoxide radical: an endogenous toxicant. Ann. Rev. Pharmacol. Toxicol. 23, 239–257 (1983).
Finkel, T. & Holbrook, N. J. Oxidants, oxidative stress and the biology of aging. Nature 408, 239–244 (2000).
Ames, B. N., Shigenaga, M. K. & Hagen, T. M. Oxidants, antioxidants and the degenerative diseases of aging. Proc. Natl Acad. Sci. USA 90, 7915–7922 (1993).
McCord, J. M. Superoxide dismutase in aging and disease: an overview. Methods Enzymol. 349, 331–341 (2002).
Nicholson, D. W. et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376, 37–43 (1995).
Porter, A. G. & Janicke, R. U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6, 99–104 (1999).
Oliver, L. & Vallette, F. M. The role of caspases in cell death and differentiation. Drug Resist. Updates 8, 163–170 (2005).
Caron, N. J. et al. Intracellular delivery of a Tat–eGFP fusion protein into muscle cells. Mol. Ther. 3, 310–318 (2001).
Acknowledgements
This work was partially supported by the Defense Threat Reducing Agency (DTRA), NSF-CAREER, Sandia National Laboratories and CheungKong Scholar Program through Tsinghua University, China.
Author information
Authors and Affiliations
Contributions
M.Y., Z.L., T.S., Y.T. and Y.L. conceived and designed the experiments. M.Y., J.D. and Z.G. performed the experiments. Y.H., W.Z., L.W. and Z.H.Z. helped to analyse the data. Z.L., T.S., Y.T. and Y.L. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Supplementary information
Supplementary information
Supplementary information (PDF 1611 kb)
Rights and permissions
About this article
Cite this article
Yan, M., Du, J., Gu, Z. et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nature Nanotech 5, 48–53 (2010). https://doi.org/10.1038/nnano.2009.341
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2009.341
This article is cited by
-
Traceless photodegradable polymer cocoons for universal protein delivery and light-controlled gene editing
Science China Chemistry (2024)
-
Biomimetic liposomal nanozymes improve breast cancer chemotherapy with enhanced penetration and alleviated hypoxia
Journal of Nanobiotechnology (2023)
-
Protein transfection via spherical nucleic acids
Nature Protocols (2022)
-
Protein nanocapsules based vectors for efficient gene transfection
Nano Research (2022)
-
Biomedical polymers: synthesis, properties, and applications
Science China Chemistry (2022)