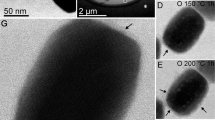
Abstract
Magnetotactic bacteria biomineralize iron into magnetite (Fe3O4) nanoparticles that are surrounded by lipid vesicles. These ‘magnetosomes’ have considerable potential for use in bio- and nanotechnological applications because of their narrow size and shape distribution and inherent biocompatibility1,2,3. The ability to tailor the magnetic properties of magnetosomes by chemical doping would greatly expand these applications4,5; however, the controlled doping of magnetosomes has so far not been achieved. Here, we report controlled in vivo cobalt doping of magnetosomes in three strains of the bacterium Magnetospirillum. The presence of cobalt increases the coercive field of the magnetosomes—that is, the field necessary to reverse their magnetization—by 36–45%, depending on the strain and the cobalt content. With elemental analysis, X-ray absorption and magnetic circular dichroism, we estimate the cobalt content to be between 0.2 and 1.4%. These findings provide an important advance in designing biologically synthesized nanoparticles with useful highly tuned magnetic properties.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bauerlein, E. Biomineralization of unicellular organisms: An unusual membrane biochemistry for the production of inorganic nano- and microstructures. Angew. Chem. Int. Edn 42, 614–641 (2003).
Lang, C., Schueler, D. & Faivre, D. Synthesis of magnetite nanoparticles for bio- and nanotechnology: Genetic engineering and biomimetics of bacterial magnetosomes. Macromol. Biosci. 7, 144–151 (2007).
Devouard, B. et al. Magnetite from magnetotactic bacteria; size distributions and twinning. Am. Mineral. 83, 1387–1398 (1998).
Lang, C. & Schueler, D. Biogenic nanoparticles: Production, characterization, and application of bacterial magnetosomes. J. Phys.: Condens. Matter 18, S2815–S2828 (2006).
Bazylinski, D. A. Controlled biomineralization of magnetic minerals by magnetotactic bacteria. Chem. Geol. 132, 191–198 (1996).
Krishnan, K. et al. Nanomagnetism and spin electronics: materials, microstructure and novel properties. J. Mater. Sci. 41, 793–815 (2006).
Mehta, R. V. & Upadhyay, R. V. Science and technology of ferrofluids. Curr. Sci. 76, 305–312 (1999).
Duguet, E., Vasseur, S., Mornet, S. & Devoisselle, J.-M. Magnetic nanoparticles and their applications in medicine. Nanomedicine 1, 157–168 (2006).
Sarikaya, M., Tamerler, C., Jen, A. K. Y., Schulten, K. & Baneyx, F. Molecular biomimetics: Nanotechnology through biology. Nature Mater. 2, 577–585 (2003).
Schuler, D. & Baeuerlein, E. Dynamics of iron uptake and Fe3O4 biomineralization during aerobic and microaerobic growth of Magnetospirillum gryphiswaldense. J. Bacteriol. 180, 159–162 (1998).
Suzuki, T. et al. Global gene expression analysis of iron-inducible genes in Magnetospirillum magneticum AMB-1. J. Bacteriol. 188, 2275–2279 (2006).
Schuler, D. & Baeuerlein, E. Iron-limited growth and kinetics of iron uptake in Magnetospirillum gryphiswaldense. Arch. Microbiol. 166, 301–307 (1996).
Towe, K. M. & Moench, T. T. Electron-optical characterization of bacterial magnetite. Earth Planet. Sci. Lett. 52, 213–220 (1981).
Bazylinski, D. A., Garratt-Reed, A. J., Abedi, A. & Frankel, R. B. Copper association with iron sulfide magnetosomes in a magnetotactic bacterium. Arch. Microbiol. 160, 35–42 (1993).
Li, R. et al. A study of the magnetotactic bacteria and their magnetosomes from a section of loess in the Shanxi Province, China. Diqiu Huaxue 25, 251–254 (1996).
Gorby, Y. A. Regulation of magnetosome biogenesis by oxygen and nitrogen. PhD thesis, Univ. New Hampshire (1989).
Coker, V. S. Formation of magnetic nanoparticles by Fe(III)-reducing bacteria: synthesis and characterisation. PhD thesis, Univ. Manchester (2007).
Pattrick, R. A. D. et al. Cation site occupancy in spinel ferrites studied by X-ray magnetic circular dichroism: Developing a method for mineralogists. Eur. J. Mineral. 14, 1095–1102 (2002).
Coker, V. S. et al. XAS and XMCD evidence for species-dependent partitioning of arsenic during microbial reduction of ferrihydrite to magnetite. Environ. Sci. Technol. 40, 7745–7750 (2006).
Komeili, A., Vali, H., Beveridge, T. J. & Newman, D. K. Magnetosome vesicles are present before magnetite formation, and MamA is required for their activation. Proc. Natl Acad. Sci. USA 101, 3839–3844 (2004).
Staniland, S. S., Ward, B., Harrison, A., van der Laan, G. & Telling, N. D. Rapid magnetosome formation shown by real-time X-ray magnetic circular dichroism. Proc. Natl Acad. Sci. USA 104, 19524–19528 (2007).
Dunlop, D. J. & Özdemir, Ö. Rock Magnetism: Fundamentals and Frontiers (Cambridge Univ. Press, Cambridge, 1997).
Pan, Y. et al. Rock magnetic properties of uncultured magnetotactic bacteria. Earth Planet. Sci. Lett. 237, 311–325 (2005).
Prozorov, R. et al. Magnetic irreversibility and the Verwey transition in nanocrystalline bacterial magnetite. Phys. Rev. B 76, 054406 (2007).
Brabers, V. A. M., Walz, F. & Kronmuller, H. Impurity effects upon the Verwey transition in magnetite. Phys. Rev. B 58, 14163–14166 (1998).
Dunin-Borkowski, R. E. et al. Magnetic microstructure of magnetotactic bacteria by electron holography. Science 282, 1868–1870 (1998).
Moskowitz, B. M., Frankel, R. B., Bazylinski, D. A., Jannasch, H. W. & Lovley, D. R. A comparison of magnetite particles produced anaerobically by magnetotactic and dissimilatory iron-reducing bacteria. Geophys. Res. Lett. 16, 665–668 (1989).
Penninga, I., de Waard, H., Moskowitz, B. M., Bazylinski, D. A. & Frankel, R. B. Remanence measurements on individual magnetotactic bacteria using a pulsed magnetic field. J. Magn. Magn. Mater. 149, 279–286 (1995).
Williams, W. & Dunlop, D. J. Some effects of grain shape and varying external magnetic fields on the magnetic structure of small grains of magnetite. Phys. Earth Planet. Int. 65, 1–14 (1990).
Dhara, S., Kotnala, R. K., Rastogi, A. C. & Das, B. K. Magnetic properties of chemical vapor deposited cobalt doped gamma iron oxide thin films. Jpn. J. Appl. Phys. 1 31, 3853–3857 (1992).
Schultheiss, D. & Schuler, D. Development of a genetic system for Magnetospirillum gryphiswaldense. Arch. Microbiol. 179, 89–94 (2003).
Grunberg, K., Wawer, C., Tebo, B. M. & Schuler, D. A large gene cluster encoding several magnetosome proteins is conserved in different species of magnetotactic bacteria. Appl. Environ. Microbiol. 67, 4573–4582 (2001).
Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (Grant no. EP/C53204X/1 & EP/D057310/1). We would like to thank L. Eades for technical support with ICP-OES, as well as C. How, M. Tanaka and M. Jaffer for support with TEM. We thank A. Muxworthy for assistance and discussions regarding the computational data and V. S. Coker for useful discussions and advice about the XMCD data. We are also grateful to the staff of the Daresbury Laboratory for their support during our experiment there.
Author information
Authors and Affiliations
Contributions
S.S.S. and A.H. conceived the idea for the study. S.S.S., A.H. and F.B.W. designed the study. S.S.S. and F.B.W. optimized the bacterial growth; S.S.S. performed the bacterial preparations, magnetosome extractions, TEM, magnetic and ICP-OES experiments and analysis. N.D.T., G.v.d.L., A.H. and S.S.S. designed the XMCD study and S.S.S., F.B.W and N.D.T. performed the XMCD experiment. N.D.T and G.v.d.L. analysed the XMCD data and produced Fig. 2. W.W. performed all the computational calculation and analysis. S.S.S. composed the manuscript with contributions from all the authors. All the authors discussed the results and commented on the manuscript.
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Staniland, S., Williams, W., Telling, N. et al. Controlled cobalt doping of magnetosomes in vivo. Nature Nanotech 3, 158–162 (2008). https://doi.org/10.1038/nnano.2008.35
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2008.35
This article is cited by
-
Renaissance for magnetotactic bacteria in astrobiology
The ISME Journal (2023)
-
In vitro magnetosome remineralization for silver-magnetite hybrid magnetosome biosynthesis and used for healing of the infected wound
Journal of Nanobiotechnology (2022)
-
Magnetosomes could be protective shields against metal stress in magnetotactic bacteria
Scientific Reports (2020)
-
Transformation between nanosheets and nanowires structure in MnO2 upon providing Co2+ ions and applications for microwave absorption
Nano Research (2020)
-
Synthesis of intracellular cobalt ferrite nanocrystals by extreme acidophilic archaea Ferroplasma thermophilum
Journal of Central South University (2020)