Abstract
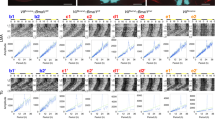
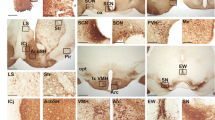
In the suprachiasmatic nucleus (SCN), the master circadian pacemaker, neurons show circadian variations in firing frequency. There is also considerable synchrony of spiking across SCN neurons on a scale of milliseconds, but the mechanisms are poorly understood. Using paired whole-cell recordings, we have found that many neurons in the rat SCN communicate via electrical synapses. Spontaneous spiking was often synchronized in pairs of electrically coupled neurons, and the degree of this synchrony could be predicted from the magnitude of coupling. In wild-type mice, as in rats, the SCN contained electrical synapses, but electrical synapses were absent in connexin36-knockout mice. The knockout mice also showed dampened circadian activity rhythms and a delayed onset of activity during transition to constant darkness. We suggest that electrical synapses in the SCN help to synchronize its spiking activity, and that such synchrony is necessary for normal circadian behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Moore, R.Y. & Eichler, V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42, 201–206 (1972).
Stephan, F.K. & Zucker, I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA 69, 1583–1586 (1972).
Lehman, M.N. et al. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J. Neurosci. 7, 1626–1638 (1987).
Ralph, M.R., Foster, R.G., Davis, F.C. & Menaker, M. Transplanted suprachiasmatic nucleus determines circadian period. Science 247, 975–978 (1990).
Berson, D.M., Dunn, F.A. & Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 (2002).
Aston-Jones, G., Chen, S., Zhu, Y. & Oshinsky, M.L. A neural circuit for circadian regulation of arousal. Nat. Neurosci. 4, 732–738 (2001).
Teclemariam-Mesbah, R., Ter Horst, G.J., Postema, F., Wortel, J. & Buijs, R.M. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. J. Comp. Neurol. 406, 171–182 (1999).
Brandstaetter, R. Circadian lessons from peripheral clocks: is the time of the mammalian pacemaker up? Proc. Natl. Acad. Sci. USA 101, 5699–5700 (2004).
LeSauter, J. & Silver, R. Output signals of the SCN. Chronobiol. Int. 15, 535–550 (1998).
Schwartz, W.J. & Gainer, H. Suprachiasmatic nucleus: use of 14C-labeled deoxyglucose uptake as a functional marker. Science 197, 1089–1091 (1977).
Inouye, S.T. & Kawamura, H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA 76, 5962–5966 (1979).
Shibata, S., Oomura, Y., Kita, H. & Hattori, K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res. 247, 154–158 (1982).
Groos, G. & Hendriks, J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci. Lett. 34, 283–288 (1982).
Prosser, R.A. & Gillette, M.U. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J. Neurosci. 9, 1073–1081 (1989).
Welsh, D.K., Logothetis, D.E., Meister, M. & Reppert, S.M. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14, 697–706 (1995).
van den Pol, A.N. Gamma-aminobutyrate, gastrin releasing peptide, serotonin, somatostatin, and vasopressin: ultrastructural immunocytochemical localization in presynaptic axons in the suprachiasmatic nucleus. Neuroscience 17, 643–659 (1986).
Strecker, G.J., Wuarin, J.P. & Dudek, F.E. GABAA-mediated local synaptic pathways connect neurons in the rat suprachiasmatic nucleus. J. Neurophysiol. 78, 2217–2220 (1997).
Wagner, S., Castel, M., Gainer, H. & Yarom, Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature 387, 598–603 (1997).
Abrahamson, E.E. & Moore, R.Y. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 916, 172–191 (2001).
Bouskila, Y. & Dudek, F.E. Neuronal synchronization without calcium-dependent synaptic transmission in the hypothalamus. Proc. Natl. Acad. Sci. USA 90, 3207–3210 (1993).
Schwartz, W.J., Gross, R.A. & Morton, M.T. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc. Natl. Acad. Sci. USA 84, 1694–1698 (1987).
Shibata, S. & Moore, R.Y. Tetrodotoxin does not affect circadian rhythms in neuronal activity and metabolism in rodent suprachiasmatic nucleus in vitro. Brain Res. 606, 259–266 (1993).
Yamaguchi, S. et al. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302, 1408–1412 (2003).
Connors, B.W. & Long, M.A. Electrical synapses in the mammalian brain. Annu. Rev. Neurosci. 27, 393–418 (2004).
Jiang, Z.G., Yang, Y.Q. & Allen, C.N. Tracer and electrical coupling of rat suprachiasmatic nucleus neurons. Neuroscience 77, 1059–1066 (1997).
Colwell, C.S. Rhythmic coupling among cells in the suprachiasmatic nucleus. J. Neurobiol. 43, 379–388 (2000).
Gibson, J.R., Beierlein, M. & Connors, B.W. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402, 75–79 (1999).
Landisman, C.E. et al. Electrical synapses in the thalamic reticular nucleus. J. Neurosci. 22, 1002–1009 (2002).
Long, M.A., Deans, M.R., Paul, D.L. & Connors, B.W. Rhythmicity without synchrony in the electrically uncoupled inferior olive. J. Neurosci. 22, 10898–10905 (2002).
Schaap, J. et al. Neurons of the rat suprachiasmatic nucleus show a circadian rhythm in membrane properties that is lost during prolonged whole-cell recording. Brain Res. 815, 154–166 (1999).
Deans, M.R., Gibson, J.R., Sellitto, C., Connors, B.W. & Paul, D.L. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron 31, 477–485 (2001).
Amitai, Y. et al. The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J. Neurosci. 22, 4142–4152 (2002).
Long, M.A., Landisman, C.E. & Connors, B.W. Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus. J. Neurosci. 24, 341–349 (2004).
Blatow, M. et al. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron 38, 805–817 (2003).
Chu, Z., Galarreta, M. & Hestrin, S. Synaptic interactions of late-spiking neocortical neurons in layer 1. J. Neurosci. 23, 96–102 (2003).
Pennartz, C.M., De Jeu, M.T., Geurtsen, A.M., Sluiter, A.A. & Hermes, M.L. Electrophysiological and morphological heterogeneity of neurons in slices of rat suprachiasmatic nucleus. J. Physiol. (Lond.) 506, 775–793 (1998).
Shinohara, K., Funabashi, T., Nakamura, T.J. & Kimura, F. Effects of estrogen and progesterone on the expression of connexin-36 mRNA in the suprachiasmatic nucleus of female rats. Neurosci. Lett. 309, 37–40 (2001).
Pennartz, C.M., de Jeu, M.T., Bos, N.P., Schaap, J. & Geurtsen, A.M. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature 416, 286–290 (2002).
Christie, M.J., Williams, J.T. & North, R.A. Electrical coupling synchronizes subthreshold activity in locus coeruleus neurons in vitro from neonatal rats. J. Neurosci. 9, 3584–3589 (1989).
Grace, A.A. & Bunney, B.S. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–3. Evidence for electrotonic coupling. Neuroscience 10, 333–348 (1983).
Perez-Armendariz, M., Roy, C., Spray, D.C. & Bennett, M.V. Biophysical properties of gap junctions between freshly dispersed pairs of mouse pancreatic beta cells. Biophys. J. 59, 76–92 (1991).
Kramer, A. et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science 294, 2511–2515 (2001).
Cheng, M.Y. et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417, 405–410 (2002).
Harris, A.L. Emerging issues of connexin channels: biophysics fills the gap. Q. Rev. Biophys. 34, 325–472 (2001).
Srinivas, M. et al. Functional properties of channels formed by the neuronal gap junction protein connexin36. J. Neurosci. 19, 9848–9855 (1999).
Kistler, W.M. et al. Analysis of Cx36 knockout does not support tenet that olivary gap junctions are required for complex spike synchronization and normal motor performance. Ann. NY Acad. Sci. 978, 391–404 (2002).
Guldenagel, M. et al. Visual transmission deficits in mice with targeted disruption of the gap junction gene connexin36. J. Neurosci. 21, 6036–6044 (2001).
Deans, M.R., Volgyi, B., Goodenough, D.A., Bloomfield, S.A. & Paul, D.L. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron 36, 703–712 (2002).
Lupi, D. et al. Transgenic ablation of rod photoreceptors alters the circadian phenotype of mice. Neuroscience 89, 363–374 (1999).
Sokolove, P.G. & Bushell, W.N. The chi square periodogram: its utility for analysis of circadian rhythms. J. Theor. Biol. 72, 131–160 (1978).
Acknowledgements
We thank S. Patrick for technical help, A. Jackson, M. Carskadon and D. Berson for comments on the manuscript, and M. Deans and D. Paul for the Cx36-knockout mouse line. This research was supported by a Sidney A. Fox and Dorothea Doctors Fox Postdoctoral Fellowship to M.A.L. and by US National Institutes of Health grants MH60284 to R.D.B. and NS25983 and DA12500 to B.W.C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Long, M., Jutras, M., Connors, B. et al. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci 8, 61–66 (2005). https://doi.org/10.1038/nn1361
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1361
This article is cited by
-
Connexin30 and Connexin43 show a time-of-day dependent expression in the mouse suprachiasmatic nucleus and modulate rhythmic locomotor activity in the context of chronodisruption
Cell Communication and Signaling (2019)
-
Constant light enhances synchrony among circadian clock cells and promotes behavioral rhythms in VPAC2-signaling deficient mice
Scientific Reports (2015)