Abstract
Initiation of drinking behavior relies on both internal state and peripheral water detection. While central neural circuits regulating thirst have been well studied, it is still unclear how mammals recognize external water. Here we show that acid-sensing taste receptor cells (TRCs) that were previously suggested as the sour taste sensors also mediate taste responses to water. Genetic silencing of these TRCs abolished water-evoked responses in taste nerves. Optogenetic self-stimulation of acid-sensing TRCs in thirsty animals induced robust drinking responses toward light even without water. This behavior was only observed when animals were water-deprived but not under food- or salt-depleted conditions, indicating that the hedonic value of water-evoked responses is highly internal-state dependent. Conversely, thirsty animals lacking functional acid-sensing TRCs showed compromised discrimination between water and nonaqueous fluids. Taken together, this study revealed a function of mammalian acid-sensing TRCs that provide a cue for external water.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
McKinley, M.J. & Johnson, A.K. The physiological regulation of thirst and fluid intake. News Physiol. Sci. 19, 1–6 (2004).
Sternson, S.M. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron 77, 810–824 (2013).
Abbott, S.B., Machado, N.L., Geerling, J.C. & Saper, C.B. Reciprocal control of drinking behavior by median preoptic neurons in mice. J. Neurosci. 36, 8228–8237 (2016).
McKinley, M.J. et al. The sensory circumventricular organs of the mammalian brain. Adv. Anat. Embryol. Cell Biol. 172, 1–122 (2003).
Oka, Y., Ye, M. & Zuker, C.S. Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature 520, 349–352 (2015).
Stricker, E.M. & Sved, A.F. Thirst. Nutrition 16, 821–826 (2000).
Young, J.K. Hunger, Thirst, Sex, and Sleep: How the Brain Controls Our Passions (Rowman & Littlefield, 2012).
Chaudhari, N. & Roper, S.D. The cell biology of taste. J. Cell Biol. 190, 285–296 (2010).
Finger, T.E. Evolution of taste and solitary chemoreceptor cell systems. Brain Behav. Evol. 50, 234–243 (1997).
Liman, E.R., Zhang, Y.V. & Montell, C. Peripheral coding of taste. Neuron 81, 984–1000 (2014).
Yarmolinsky, D.A., Zuker, C.S. & Ryba, N.J. Common sense about taste: from mammals to insects. Cell 139, 234–244 (2009).
Chandrashekar, J. et al. The cells and peripheral representation of sodium taste in mice. Nature 464, 297–301 (2010).
Shigemura, N. et al. Amiloride-sensitive NaCl taste responses are associated with genetic variation of ENaC alpha-subunit in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R66–R75 (2008).
Matsunami, H., Montmayeur, J.P. & Buck, L.B. A family of candidate taste receptors in human and mouse. Nature 404, 601–604 (2000).
Mueller, K.L. et al. The receptors and coding logic for bitter taste. Nature 434, 225–229 (2005).
Nelson, G. et al. An amino-acid taste receptor. Nature 416, 199–202 (2002).
Nelson, G. et al. Mammalian sweet taste receptors. Cell 106, 381–390 (2001).
Huang, A.L. et al. The cells and logic for mammalian sour taste detection. Nature 442, 934–938 (2006).
Ishimaru, Y. et al. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA 103, 12569–12574 (2006).
Ye, W. et al. The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc. Natl. Acad. Sci. USA 113, E229–E238 (2016).
Evans, D.R. & Mellon, D. Jr. Electrophysiological studies of a water receptor associated with the taste sensilla of the blow-fly. J. Gen. Physiol. 45, 487–500 (1962).
Wolbarsht, M.L. Water taste in Phormia. Science 125, 1248 (1957).
Cameron, P., Hiroi, M., Ngai, J. & Scott, K. The molecular basis for water taste in Drosophila. Nature 465, 91–95 (2010).
Liljestrand, G. & Zotterman, Y. The water taste in mammals. Acta Physiol. Scand. 32, 291–303 (1954).
Shingai, T. Ionic mechanism of water receptors in the laryngeal mucosa of the rabbit. Jpn. J. Physiol. 27, 27–42 (1977).
Shingai, T. & Beidler, L.M. Response characteristics of three taste nerves in mice. Brain Res. 335, 245–249 (1985).
Rosen, A.M., Roussin, A.T. & Di Lorenzo, P.M. Water as an independent taste modality. Front. Neurosci. 4, 175 (2010).
Edgar, W.M. & O'Mullane, D.M. Saliva and Oral Health (British Dental Association, 1996).
Schneyer, L.H., Young, J.A. & Schneyer, C.A. Salivary secretion of electrolytes. Physiol. Rev. 52, 720–777 (1972).
Zhang, Y. et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301 (2003).
Halpern, B.P. Amiloride and vertebrate gustatory responses to NaCl. Neurosci. Biobehav. Rev. 23, 5–47 (1998).
Chandrashekar, J. et al. The taste of carbonation. Science 326, 443–445 (2009).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Zald, D.H. & Pardo, J.V. Cortical activation induced by intraoral stimulation with water in humans. Chem. Senses 25, 267–275 (2000).
Bushman, J.D., Ye, W. & Liman, E.R. A proton current associated with sour taste: distribution and functional properties. FASEB J. 29, 3014–3026 (2015).
Hanamori, T. Effects of various ion transport inhibitors on the water response in the superior laryngeal nerve in rats. Chem. Senses 26, 897–903 (2001).
Oka, Y., Butnaru, M., von Buchholtz, L., Ryba, N.J. & Zuker, C.S. High salt recruits aversive taste pathways. Nature 494, 472–475 (2013).
Chang, R.B., Waters, H. & Liman, E.R. A proton current drives action potentials in genetically identified sour taste cells. Proc. Natl. Acad. Sci. USA 107, 22320–22325 (2010).
Dessirier, J.M., O'Mahony, M., Iodi-Carstens, M. & Carstens, E. Sensory properties of citric acid: psychophysical evidence for sensitization, self-desensitization, cross-desensitization and cross-stimulus-induced recovery following capsaicin. Chem. Senses 25, 769–780 (2000).
Finger, T.E. et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310, 1495–1499 (2005).
Peng, Y. et al. Sweet and bitter taste in the brain of awake behaving animals. Nature 527, 512–515 (2015).
Betley, J.N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015).
Baliga, S., Muglikar, S. & Kale, R. Salivary pH: a diagnostic biomarker. J. Indian Soc. Periodontol. 17, 461–465 (2013).
Yamamoto, M. et al. Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J. Neurosci. 23, 6759–6767 (2003).
Storm, J.F. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J. Physiol. (Lond.) 385, 733–759 (1987).
Acknowledgements
We thank B. Ho for help with mouse husbandry. We also thank K. Scott, M. Meister and D.J. Anderson for helpful suggestions. We thank C.S. Zuker (Columbia) and N. Ryba (NIDCR) for generously sharing Pkd2l1-Cre and TRPM5 knockout transgenic animals, H. Matsunami (Duke) for PKD2L1 antibody, S. Lee for technical support and members of the Oka laboratory for comments. This work was supported by Startup funds from the President and Provost of California Institute of Technology and the Biology and Biological Engineering Division of California Institute of Technology. Y.O. is also supported by the Searle Scholars Program, the Mallinckrodt Foundation, the Okawa Foundation, the McKnight Foundation and the Klingenstein-Simons Foundation. Support was provided by DFG WE 2344/9-1 to G.W. Y.O. have disclosed these methods and findings to the Caltech Office of Technology Transfer.
Author information
Authors and Affiliations
Contributions
D.Z. and Y.O. conceived the research program. D.Z. and Y.O. designed and carried out the experiments and analyzed data. G.W. maintained and provided CA4 knockout animals. D.Z. analyzed data and, together with Y.O., wrote the paper. Y.O. supervised the entire work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Ionic effects on taste responses induced by water
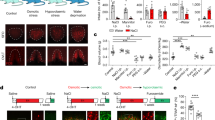
a, Effect of sodium ions on water responses. Representative traces of water responses after NaCl are shown in the presence or absence of amiloride (Ami), a blocker of the epithelium sodium channel (left). A reduction of nerve firing by the removal of NaCl is completely blocked by amiloride, suggesting that this change is mediated by the sodium taste receptor. Quantified nerve responses are shown (n=4 for NaCl + Ami). The data for NaCl alone is from Figure 1b for reference. b, Representative water responses induced by the removal of phosphate ions. In addition to bicarbonate ions, washing out of high concentrations of phosphate (KH2PO4) induced minor responses.
Supplementary Figure 2 Taste responses in Trpm5−/− and Pdk2l1TeNT mice
a, Knocking out of TRPM5 has no effect on salt and sour responses. Nerve responses to salt (60 mM NaCl) in TRPM5 −/− mice were comparable to those in TRPM5 +/− control mice (n=4 for TRPM5 −/− and n=4 for TRPM5 +/−). Responses were normalized to 10 mM Citric Acid. b, Sour and water responses were specifically disrupted in PKDTeNT mice. However, response amplitudes to bitter (0.1 mM cycloheximide), salt (60 mM NaCl), umami (50 mM MPG + 1 mM IMP), and sweet (8 mM AceK) were similar between PKDTeNT (n=6) and TeNT control mice (n=5). Responses were normalized to 8 mM AceK. Data were analyzed with two-tailed Mann-Whitney U-test. Values are means ± s.e.m
Supplementary Figure 3 Carbonic anhydrase–independent taste responses and the kinetics of PKD2L1 taste responses
a, CA4 knockout mice exhibit significantly reduced responses to non-buffered water (n=9 for CA4 −/−, n=8 for CA4 −/+; p=0.0464). All other tastants evoked similar response magnitudes in both genotypes (n=6 for CA4 −/−, n=4 for CA4 +/−). b, Treatments with dorzolamide (DZA) or benzolamide (BZA) had no effect on basic taste responses (n=3). c, A proposed model for activation of acid-sensing TRCs by water and sour. Acids (protons) directly activate PKD2L1-expressing TRCs through putative proton/potassium channels45. On the other hand, washing out of bicarbonate with water drives catalytic reaction of CA in PKD2L1-expressing TRCs, leading to increase in local protons. d, Representative taste nerve responses to water and citric acid from the same animal (left). Response rise time (n=7, p=0.0157, middle), and ratio of the rising slopes (right) show slower kinetics of water responses compared to citric acid. Data were analyzed with two-tailed paired t-test. Values are means ± s.e.m
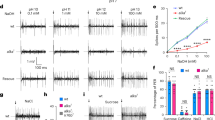
Supplementary Figure 4 Light-induced taste nerve responses in Pdk2l1ChR2 mice
a, The tongue was stimulated with laser pulses (8 Hz, 40 ms duration) at 48 mW for 2 s. Shown is a representative trace of three sets of pulse trains. Inset shows a magnified view of a 2-s stimulation window. Each blue triangle corresponds to a laser pulse. An increase in population activity in the nerve is precisely time-locked to laser pulses. b, Total number of licks induced by different levels of laser power. The number of licks was summed during a 1-min session (n=5). Each data point was obtained and averaged from three PKD2L1ChR2 animals. Values are means ± s.e.m.
Supplementary Figure 5 Ectopic expression of ChR2-EYFP in the geniculate ganglion
Tissue staining of taste buds in the circumvallate papillae (top), and geniculate ganglion (secondary taste station, bottom) from a PKD2L1ChR2 animal. Shown are representative staining of ChR2-EYFP (labeled with anti-GFP antibody, left), co-labeled with anti-PKD2L1 antibody (middle); the right panels show merged images. ChR2-EYFP signals overlap with PKD2L1 expression in taste buds (top panel). However, ectopic expressions of ChR2-EYFP in geniculate neurons (arrow heads, bottom left) do not show PKD2L1 expression (bottom middle). Scale bars, 100 μm.
Supplementary Figure 6 Acid-sensing TRCs are important for fluid discrimination, but not for water consumption
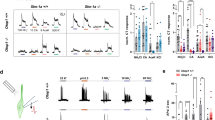
a, Plots of drinking behavior of PKD2L1TeNT and control mice during 5-min consumption tests. Either water or silicone oil was presented to each animal for 5 min after 23 h water-deprivation regime. Individual black bars indicate each lick event. Average number of licks are quantified for each 10-sec period (bottom). b, Cumulative number of licks is shown during the 5-min sessions (n=4, p=0.0188 at 5 min). c, A role of taste pathway for discriminating water and mineral oil. To test if animals can discriminate water and mineral oil, water was first presented to water-deprived animals for 5 s (water), followed by 5 consecutive presentations of mineral oils (5 s each, 1-5 trials). Consistent with the results of silicone oil, PKD2L1TeNT initially consumed comparable amount of mineral oil to water (1-3 trials), but animals learned to discriminate in later trials (4-5 trials, n=3, p=0.0467, water vs 4-5 trials) possibly using other sensory cues such as olfaction and tactile. By contrast, control (TeNT) mice preferred water over mineral oil throughout the trials (n=4, p=0.0012, water vs 1-3 trials, p=0.0007, water vs all). Data were analyzed with two-tailed paired t-test. Values are means ± s.e.m
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 655 kb)
Rights and permissions
About this article
Cite this article
Zocchi, D., Wennemuth, G. & Oka, Y. The cellular mechanism for water detection in the mammalian taste system. Nat Neurosci 20, 927–933 (2017). https://doi.org/10.1038/nn.4575
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4575
This article is cited by
-
The proton channel OTOP1 is a sensor for the taste of ammonium chloride
Nature Communications (2023)
-
Sensory representation and detection mechanisms of gut osmolality change
Nature (2022)
-
How to Manage Taste Disorders
Current Otorhinolaryngology Reports (2022)
-
Durst und Trinken – Physiologie und Bedeutung für die Störungen des Wasserhaushalts
Journal für Klinische Endokrinologie und Stoffwechsel (2022)
-
Satb2 neurons in the parabrachial nucleus mediate taste perception
Nature Communications (2021)