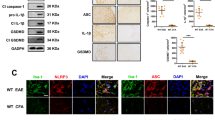
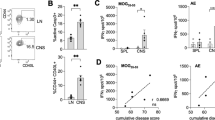
Abstract
Inflammation induced by innate immunity influences the development of T cell–mediated autoimmunity in multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis (EAE). We found that strong activation of innate immunity induced Nod-like receptor protein 3 (NLRP3) inflammasome–independent and interferon-β (IFNβ)-resistant EAE (termed type B EAE), whereas EAE induced by weak activation of innate immunity requires the NLRP3 inflammasome and is sensitive to IFNβ treatment. Instead, an alternative inflammatory mechanism, including membrane-bound lymphotoxin-β receptor (LTβR) and CXC chemokine receptor 2 (CXCR2), is involved in type B EAE development, and type B EAE is ameliorated by antagonizing these receptors. Relative expression of Ltbr and Cxcr2 genes was indeed enhanced in patients with IFNβ-resistant multiple sclerosis. Remission was minimal in type B EAE due to neuronal damages induced by semaphorin 6B upregulation on CD4+ T cells. Our data reveal a new inflammatory mechanism by which an IFNβ-resistant EAE subtype develops.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Haines, J.D., Inglese, M. & Casaccia, P. Axonal damage in multiple sclerosis. Mt. Sinai J. Med. 78, 231–243 (2011).
Río, J. et al. Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Ann. Neurol. 59, 344–352 (2006).
Rivers, T.M., Sprunt, D.H. & Berry, G.P. Observations on attempts to produce acute disseminated encephalomyelitis in monkeys. J. Exp. Med. 58, 39–53 (1933).
Arnon, R. & Sela, M. Immunomodulation by the copolymer glatiramer acetate. J. Mol. Recognit. 16, 412–421 (2003).
Steinman, L. The discovery of natalizumab, a potent therapeutic for multiple sclerosis. J. Cell Biol. 199, 413–416 (2012).
Simmons, S.B., Pierson, E.R., Lee, S.Y. & Goverman, J.M. Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol. 34, 410–422 (2013).
Inoue, M. et al. Interferon-β therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci. Signal. 5, ra38 (2012).
Inoue, M., Williams, K.L., Gunn, M.D. & Shinohara, M.L. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 109, 10480–10485 (2012).
Gris, D. et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J. Immunol. 185, 974–981 (2010).
Shaw, P.J. et al. Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J. Immunol. 184, 4610–4614 (2010).
Smyk, D.S., Alexander, A.K., Walker, M. & Walker, M. Acute disseminated encephalomyelitis progressing to multiple sclerosis: are infectious triggers involved? Immunol. Res. 60, 16–22 (2014).
Meeker, R.B., Williams, K., Killebrew, D.A. & Hudson, L.C. Cell trafficking through the choroid plexus. Cell Adh. Migr. 6, 390–396 (2012).
Steinman, L. Some misconceptions about understanding autoimmunity through experiments with knockouts. J. Exp. Med. 185, 2039–2041 (1997).
Lock, C., Oksenberg, J. & Steinman, L. The role of TNFalpha and lymphotoxin in demyelinating disease. Ann. Rheum. Dis. 58 (Suppl. 1), I121–I128 (1999).
Woodroofe, M.N. & Cuzner, M.L. Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine 5, 583–588 (1993).
Kraus, J. et al. Serum cytokine levels do not correlate with disease activity and severity assessed by brain MRI in multiple sclerosis. Acta Neurol. Scand. 105, 300–308 (2002).
Columba-Cabezas, S. et al. Suppression of established experimental autoimmune encephalomyelitis and formation of meningeal lymphoid follicles by lymphotoxin beta receptor-Ig fusion protein. J. Neuroimmunol. 179, 76–86 (2006).
Axtell, R.C. et al. T helper type 1 and 17 cells determine efficacy of interferon-β in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 16, 406–412 (2010).
Codarri, L. et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567 (2011).
Wang, C. et al. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163, 1413–1427 (2015).
Bustamante, M.F., Nurtdinov, R.N., Río, J., Montalban, X. & Comabella, M. Baseline gene expression signatures in monocytes from multiple sclerosis patients treated with interferon-beta. PLoS One 8, e60994 (2013).
Roth, G.A., Spada, V., Hamill, K. & Bornstein, M.B. Insulin-like growth factor I increases myelination and inhibits demyelination in cultured organotypic nerve tissue. Brain Res. Dev. Brain Res. 88, 102–108 (1995).
McMorris, F.A., Smith, T.M., DeSalvo, S. & Furlanetto, R.W. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc. Natl. Acad. Sci. USA 83, 822–826 (1986).
Magliozzi, R. et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130, 1089–1104 (2007).
Tawarayama, H., Yoshida, Y., Suto, F., Mitchell, K.J. & Fujisawa, H. Roles of semaphorin-6B and plexin-A2 in lamina-restricted projection of hippocampal mossy fibers. J. Neurosci. 30, 7049–7060 (2010).
Rothhammer, V. et al. Th17 lymphocytes traffic to the central nervous system independently of α4 integrin expression during EAE. J. Exp. Med. 208, 2465–2476 (2011).
Liu, Y. et al. Preferential recruitment of neutrophils into the cerebellum and brainstem contributes to the atypical experimental autoimmune encephalomyelitis phenotype. J. Immunol. 195, 841–852 (2015).
Qin, H. et al. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc. Natl. Acad. Sci. USA 109, 5004–5009 (2012).
Lukens, J.R. et al. The NLRP12 sensor negatively regulates autoinflammatory disease by modulating interleukin-4 production in T cells. Immunity 42, 654–664 (2015).
Siffrin, V. et al. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity 33, 424–436 (2010).
Mo, R. et al. T cell chemokine receptor expression in aging. J. Immunol. 170, 895–904 (2003).
Liu, Y.J. et al. Peripheral T cells derived from Alzheimer's disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-alpha-dependent. Neurobiol. Aging 31, 175–188 (2010).
Pokkali, S., Das, S.D. & Logamurthy, R. Expression of CXC and CC type of chemokines and its receptors in tuberculous and non-tuberculous effusions. Cytokine 41, 307–314 (2008).
Wang, Y. et al. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity 32, 403–413 (2010).
Revesz, T., Kidd, D., Thompson, A.J., Barnard, R.O. & McDonald, W.I. A comparison of the pathology of primary and secondary progressive multiple sclerosis. Brain 117, 759–765 (1994).
Thompson, A.J. et al. Major differences in the dynamics of primary and secondary progressive multiple sclerosis. Ann. Neurol. 29, 53–62 (1991).
Suto, F. et al. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J. Neurosci. 25, 3628–3637 (2005).
Zhuang, B., Su, Y.S. & Sockanathan, S. FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling. Neuron 61, 359–372 (2009).
Suto, F. et al. Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron 53, 535–547 (2007).
Williams, A. et al. Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain 130, 2554–2565 (2007).
Liu, N.K. et al. Cytosolic phospholipase A2 protein as a novel therapeutic target for spinal cord injury. Ann. Neurol. 75, 644–658 (2014).
Kolko, M., Rodriguez de Turco, E.B., Diemer, N.H. & Bazan, N.G. Neuronal damage by secretory phospholipase A2: modulation by cytosolic phospholipase A2, platelet-activating factor, and cyclooxygenase-2 in neuronal cells in culture. Neurosci. Lett. 338, 164–168 (2003).
Takemiya, T. et al. Endothelial microsomal prostaglandin E synthase-1 facilitates neurotoxicity by elevating astrocytic Ca2+ levels. Neurochem. Int. 58, 489–496 (2011).
Whitney, L.W., Ludwin, S.K., McFarland, H.F. & Biddison, W.E. Microarray analysis of gene expression in multiple sclerosis and EAE identifies 5-lipoxygenase as a component of inflammatory lesions. J. Neuroimmunol. 121, 40–48 (2001).
Neu, I., Mallinger, J., Wildfeuer, A. & Mehlber, L. Leukotrienes in the cerebrospinal fluid of multiple sclerosis patients. Acta Neurol. Scand. 86, 586–587 (1992).
Yoshikawa, K., Palumbo, S., Toscano, C.D. & Bosetti, F. Inhibition of 5-lipoxygenase activity in mice during cuprizone-induced demyelination attenuates neuroinflammation, motor dysfunction and axonal damage. Prostaglandins Leukot. Essent. Fatty Acids 85, 43–52 (2011).
Liu, L. et al. Functional defect of peripheral neutrophils in mice with induced deletion of CXCR2. Genesis 51, 587–595 (2013).
Chiang, E.Y. et al. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat. Med. 15, 766–773 (2009).
Shinohara, M.L., Kim, J.H., Garcia, V.A. & Cantor, H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity 29, 68–78 (2008).
Shinohara, M.L. et al. Osteopontin expression is essential for interferon-α production by plasmacytoid dendritic cells. Nat. Immunol. 7, 498–506 (2006).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. Bioinform. in Action 17, 10–12 (2012).
Kersey, P.J. et al. Ensembl Genomes: an integrative resource for genome-scale data from non-vertebrate species. Nucleic Acids Res. 40, D91–D97 (2012).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Robinson, M.D., McCarthy, D.J. & Smyth, G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Gentleman, R.C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004).
Robinson, M.D. & Smyth, G.K. Small-sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 9, 321–332 (2008).
Mootha, V.K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Banker, G. & Goslin, K. Developments in neuronal cell culture. Nature 336, 185–186 (1988).
Eddy, N.B. & Leimbach, D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J. Pharmacol. Exp. Ther. 107, 385–393 (1953).
Acknowledgements
We thank A. Uezu for help in primary hippocampal neuron culture and Golgi staining, S. McKinnon for help in isolating optic nerves, M. Luftig for MHV68 experiments and M. Skeen for discussions on IFNβ treatment of MS patients. We are also grateful to S. Cote, S. Arvai, and all participants in the MURDOCK Study Community Registry and Biorepository and the MURDOCK Multiple Sclerosis Cohort Study. We thank MURDOCK Study leadership and staff, including principal investigator K. Newby, for providing samples and support. This study was funded by the National Multiple Sclerosis Society (RG4536 to M.L.S.; RG4723 to C.L.) and NIH (R01MH096979 and P41EB015897 to C.L.).
Author information
Authors and Affiliations
Contributions
M.I. and M.L.S. designed the study, analyzed data, and wrote the manuscript. M.I. performed most of the experiments. P.-h.C. performed methylation assay. S.G.G. selected RRMS patient samples from the MURDOCK study cohorts. S.S. performed qPCR analysis for RRMS patient samples. C.L. performed MRI analysis. E.B. assisted neuron imaging analyses. C.L., Q.-j.L., L.S., S.G.G. and E.B. contributed critical discussion for data and analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 EAE induced by several immunization methods and phenotypic effects on cell migration, demyelination and nociceptive sensitivity in mice with type A and type B EAE.
(a) EAE severity in Nlrp3-/- mice was evaluated. EAE was induced with 6 different methods, as indicated in Online Methods. (b) AUC between 0 and 20-dpi. Method 1 (n=4), Method 2-6 (n=5). P(Method 1 vs 2)=0.0006, t(7)=5.98, P(Method 1 vs 3) <0.0001, t(7)=12.54, P(Method 1 vs 4)=0.0027, t(7)=4.54, P(Method 1 vs 5) <0.0001, t(7)=8.15, P(Method 1 vs 6) <0.0001, t(7)=10.63. (c) Numbers of total immune cells were evaluated in the brains and spinal cords (S.C.) of mice with Type-B EAE at 17-dpi. n=4. Brain: P(WT vs Nlrp3-/-)=0.8159, t(6)=0.2433, P(WT vs Asc-/-)=0.5685, t(6)=0.6031. Spinal cord: P(WT vs Nlrp3-/-)=0.5226, t(6)=0.6787, P(WT vs Asc-/-)=0.8984, t(6)=0.1332. (d) IFNβ treatment on Nlrp3-/- mice with Type-B EAE induced with Method 6. IFNβ (3x104 unit/mouse) were i.p. injected every other day from day 0 to 8 as previously performed. (e) Time course on body weight change in Type-A and Type-B EAE. (f) Il1b mRNA levels in splenic DC from naïve, Type-A, and Type-B EAE mice at 9 dpi. P(Naive vs Type-A)=0.0059, t(4)=5.344, P(Naive vs Type-B)<0.0001, t(4)=18.55, P(Type-A vs Type-B)=0.0004, t(4)=10.83. (g,h) Levels of extracellular IL-1β (f) and p20 caspase-1 (g) in 24h splenocyte culture supernatants (n=4 for Type-A, n=5 for Type-B). P=0.0261, t(6)=2.724 (g). P=0.0020, t(6)=4.794 (h). (i) Infiltrated cell numbers in the brain and spinal cord (n=7). Brain: P(Total)=0.0028, t(12)=3.747, P(CD4)=0.018, t(12)=2.737, P(Th17)=0.0167, t(12)=2.78, P(Th1)=0.0165, t(12)=2.784, P(CD8)=0.0329, t(12)=2.41, P(B)=0.0013, t(12)=4.165, P(DC)=0.0003, t(12)=5.008, P(PMN)=0.0004, t(12)=4.912, P(Mac)=0.0027, t(12)=3.759. Spinal cord: P(Total)=0.0035, t(12)=3.621, P(CD4)=0.0002, t(12)=5.372, P(Th17)=0.0003, t(12)=4.997, P(Th1)=0.00465, t(12)=2.221, P(CD8)=0.0104, t(12)=3.032, P(B)=0.0918, t(12)=1.833, P(DC)=0.0429, t(12)=2.263, P(PMN)=0.0011, t(12)=4.29, P(Mac)=0.089, t(12)=1.849. (j) Representative LFB-stained images of brains at 17-dpi. Red arrows indicate reduced LFB intensity, i.e., reduced myelin. Scale bars, 200 μm. (k) T2 FLAIR MRI analysis of spinal cords obtained from mice at 18-dpi. Yellow arrows indicate areas of potential myelin loss. (l) Thermal sensitivity evaluated by a hot-plate test in 9-dpi mice, which did not show any EAE symptoms and motor dysfunction. n=8. P(Naive vs Type-A)=0.1524, t(14)=1.514, P(Naive vs Type-B)=0.0013, t(14)=4.026, P(Type-A vs Type-B)=0.0217, t(14)=2.582. *; p<0.05. All statistical analyses in this figure were performed by two-tailed unpaired Student’s t-test. All the experimental data and images are representatives from at least 2 similar experiments for each.
Supplementary Figure 2 mLT expression on DCs.
(a) Flow charts showing mLT expression in DC (CD11c+ gated) obtained from naïve mice, Type-A EAE mice, and Type-B EAE mice at 9-dpi. (b) Percentages of LTα+ (i.e., mLT+) macrophages, determined by flow cytometry, in DLNs of naïve mice or mice with EAE at 9-dpi. Naïve (n=4), Type-A (n=3), Type-B (n=4). P(Naive vs Type-A)=0.1340, t(5)=1.7887, P(Naive vs Type-B)=0.0024, t(6)=45.021, P(Type-A vs Type-B)=0.0268, t(5)=3.101, by two-tailed unpaired Student’s t-test. (c) Evaluating mLT expression on DCs from mice received CFA injection alone without MOG. One group received 200 μg Mtb in CFA (Mtb dosage for Type-A EAE), and another group had 400 μg Mtb in CFA twice (Mtb dosage for Type-B EAE). (n=6) P(Naive vs Type-A)=00.7939, t(6)=0.2732, P(Naive vs Type-B)=0.0040, t(6)=4.519, P(Type-A vs Type-B)=0.0095, t(6)=3.748, by two-tailed unpaired Student’s t-test. (d) Methylation analysis was carried out by bisulfite conversion on the Lta promoter in DCs from naïve mice. Methylated and unmethylated CpG were shown with black and gray boxes, respectively
Supplementary Figure 3 RNA-seq analysis.
Heatmap for differentially expressed genes in CD4+ T cells isolated from naïve, Type-A EAE mice, and Type-B EAE mice at 9-dpi. Genes and samples have been clustered using correlation distance with complete linkage. A gene is listed on the heatmap if it had a p-value <= 0.05 and a log2FC >1 or < -1.
Supplementary Figure 4 Comparison of cell populations and gene expression in mice and RRMS patients who have diseases that do not respond to IFNβ therapies.
(a) Numbers of indicated cell types obtained from DLNs and spleen on 17-dpi were evaluated between Type-A and Type-B EAE. n=4. DLNs: P(Total)=0.2073, t(6)=1.413, P(CD3)=0.8466, t(6)=0.202, P(CD4)=0.9285, t(6)=0.09353, P(CD8)=0.7413, t(6)=0.3459, P(B)=0.4141, t(6)=0.8773, P(Th17)=0.9724, t(6)=0.03605, P(Th1)=0.9017, t(6)=0.1289, P(Treg)=0.3556, t(6)=1.001. Spleen: P(Total)=0.0072, t(6)=3.99, P(CD3)=0.2537, t(6)=1.262, P(CD4)=0.1781, t(6)=1.525, P(CD8)=0.3999, t(6)=0.906, P(B)=0.1371, t(6)=1.715. (b, c) Representative flow charts (b) and proportion (c) for GM-CSF-producing Th1 and Th17 cells from Type-A and Type-B EAE at 9-dpi. n=6. Th17: P=0.6568, t(10)=0.4579, Th1: P=0.3954, t(10)=0.8879. (d) Proportion for IFNγ-, GM-CSF-, IL-22-producing and CD5L-expressing Th17 cells in spinal cords from Type-A and Type-B EAE mice at 17-dpi. Type-A (n=4), Type-B (n=5). P(IFNγ)=0.3493, t(7)=1.0037, P(GM-CSF)=0.1760, t(7)=1.505, P(Il-22)=0.2859, t(7)=1.155, P(CD5L)=0.2641, t(7)=1.214. (e) Proportions and MFI of CXCR2+ neutrophils and macrophages obtained from naïve mice and mice with either Type-A or Type-B EAE at 9-dpi. n=4. PMN %: P(Naive vs Type-A)=0.0102, t(6)=3.695, P(Naive vs Type-B)=0.0004, t(6)= 7.001, P(Type-A vs Type-B)=0.0022, t(6)=5.131. PMN MFI: P(Naive vs Type-A)=0.0777, t(6)=2.125, P(Naive vs Type-B)=0.0014, t(6)= 5.588, P(Type-A vs Type-B)=0.0090, t(6)=3.81. Macrophage %: P(Naive vs Type-A)=0.0005, t(6)=6.774, P(Naive vs Type-B)=0.0006, t(6)= 6.48, P(Type-A vs Type-B)=0.0161, t(6)=3.314. Macrophage MFI: P(Naive vs Type-A)=0.33, t(6)=1.06, P(Naive vs Type-B)=0.0001, t(6)= 11.11, P(Type-A vs Type-B)=0.0001, t(6)=10.89. (f) Flow cytometry results showing CXCR2 protein expression on the CD4+ T surface in each group (naïve mice, Type-A, or Type-B EAE mice at 9-dpi). (g) Comparison of relative gene expression levels between LTBR and CXCR1, CXCR1 and CXCR2, LTBR and CXCR2, normalized to VCAM1 expression. Total PBMCs from RRMS patients who responded to IFNβ and those who did not respond to IFNβ were compared. All statistical analyses in this figure were performed by two-tailed unpaired Student’s t-test. All the experimental data sets, except for (g), are representatives from at least 2 similar experiments for each.
Supplementary Figure 5 CXCL1 expression in spinal cords.
Shown are representative images of typical CXCL1 staining in spinal cords from naïve mice and mice with either Type-A or Type-B EAE at 9-dpi. Scale bars, 200 μm. Images are representatives from 3 similar experiments.
Supplementary Figure 6 Histology of spinal cords in mice with type A or type B EAE.
(a-e) Shown are representative images of typical staining from multiple mice. (a) H/E staining in the spinal cord of Type-A and Type-B EAE mice at 70-dpi. (b) IGF-1 staining in spinal cord of naïve mice and mice with either Type-A or Type-B EAE at 22-dpi. (c) Bielschowsky neuron staining in spinal cords of naïve mice (e), and Type-A and Type-B EAE at 30-dpi. Red arrows indicate area showing reduced staining intensity in the spinal cord. (d,e) Golgi’s silver staining in spinal cord of naïve mice (d), and Type-A and Type-B at 22-dpi (e). All scale bars in this figure except magnified figure in (e), 200 μm. All scale bars in magnified figure in (e), 50 μm. All images are representatives from 3 similar experiments.
Supplementary Figure 7 Sema6b shRNA knockdown in T cells.
Sema6b expression levels in CD4+ T cells isolated from naïve or Type-B EAE mice at 9-dpi were evaluated by qPCR. Sema6b was knocked down by shRNA in the indicated group of CD4+ T cells. Control shRNA (scrambled shRNA sequence) was used for the control group. Values shown are obtained from three independent trials using one mouse per each. n=3. P(Naive T vs Type-B T+Control shRNA)=0.0011, t(4)=8.396, P(Type-B T+Control shRNA vs Type-B T+Sema6b shRNA)=0.0051, t(4)=5.559. *; p<0.05.
Supplementary Figure 8 Schematic diagram of distinct pathology of two EAE subtypes.
Type-A EAE is NLRP3 inflammasome-dependent and IFNβ-sensitive. Type-B EAE is induced by immunization with higher doses of Mtb than Type-A EAE induction, and is NLRP3 inflammasome-independent and IFNβ-resistant. Type-B EAE can also be induced with Type-A EAE induction methods with MHV68 infection or with rLT (rLTα2β1) injection. In Type-B EAE, Lta gene expression is epigenetically induced in DCs and the expression of membrane-bound LT (mLT) on DCs are enhanced. mLT stimulate LTβR on CD4+ T cells, resulting in the upregulation of CXCR2 on CD4+ T cells. Blockade of LTβR (with LTβR-Fc) and CXCR2 (with SB225002) selectively inhibits the Type-B EAE progression. mLT is also involved in the induction of Sema6B in T cells. Sema6B causes neural damages, and this may be a reason for the prolonged and minimal remission in Type-B EAE.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1–4 (PDF 1961 kb)
Representative mouse with Type A EAE on 17-dpi.
This video shows a mouse with Type A EAE around the peak time. (MOV 31931 kb)
Representative mouse with Type B EAE on 17-dpi.
This video shows a mouse with Type B EAE around the peak time. There are no visible differences in movement and ataxia of this mouse compared to one with Type A EAE in Supp. Video 1. (MOV 42067 kb)
Rights and permissions
About this article
Cite this article
Inoue, M., Chen, Ph., Siecinski, S. et al. An interferon-β-resistant and NLRP3 inflammasome–independent subtype of EAE with neuronal damage. Nat Neurosci 19, 1599–1609 (2016). https://doi.org/10.1038/nn.4421
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4421
This article is cited by
-
Inhibiting the NLRP3 Inflammasome with MCC950 Alleviates Neurological Impairment in the Brain of EAE Mice
Molecular Neurobiology (2024)
-
Impact of intracellular innate immune receptors on immunometabolism
Cellular & Molecular Immunology (2022)
-
TH17 cells promote CNS inflammation by sensing danger signals via Mincle
Nature Communications (2022)
-
Early-life-trauma triggers interferon-β resistance and neurodegeneration in a multiple sclerosis model via downregulated β1-adrenergic signaling
Nature Communications (2021)
-
TRPV1 channel mediates NLRP3 inflammasome-dependent neuroinflammation in microglia
Cell Death & Disease (2021)