Abstract
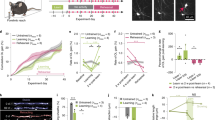
We identified mRNA encoding the ecto-enzyme Enpp6 as a marker of newly forming oligodendrocytes, and used Enpp6 in situ hybridization to track oligodendrocyte differentiation in adult mice as they learned a motor skill (running on a wheel with unevenly spaced rungs). Within just 2.5 h of exposure to the complex wheel, production of Enpp6-expressing immature oligodendrocytes was accelerated in subcortical white matter; within 4 h, it was accelerated in motor cortex. Conditional deletion of myelin regulatory factor (Myrf) in oligodendrocyte precursors blocked formation of new Enpp6+ oligodendrocytes and impaired learning within the same ∼2−3 h time frame. This very early requirement for oligodendrocytes suggests a direct and active role in learning, closely linked to synaptic strengthening. Running performance of normal mice continued to improve over the following week accompanied by secondary waves of oligodendrocyte precursor proliferation and differentiation. We concluded that new oligodendrocytes contribute to both early and late stages of motor skill learning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sturrock, R.R. Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol. 6, 415–420 (1980).
Yeung, M.S. et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774 (2014).
Dimou, L., Simon, C., Kirchhoff, F., Takebayashi, H. & Götz, M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 28, 10434–10442 (2008).
Rivers, L.E. et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392–1401 (2008).
Lasiene, J., Matsui, A., Sawa, Y., Wong, F. & Horner, P.J. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell 8, 201–213 (2009).
Kang, S.H., Fukaya, M., Yang, J.K., Rothstein, J.D. & Bergles, D.E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68, 668–681 (2010).
Zhu, X. et al. Age-dependent fate and lineage restriction of single NG2 cells. Development 138, 745–753 (2011).
Simon, C., Götz, M. & Dimou, L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia 59, 869–881 (2011).
Young, K.M. et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 (2013).
Emery, B. et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 138, 172–185 (2009).
Koenning, M. et al. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J. Neurosci. 32, 12528–12542 (2012).
Hornig, J. et al. The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet. 9, e1003907 (2013).
McKenzie, I.A. et al. Motor skill learning requires active central myelination. Science 346, 318–322 (2014).
Freeman, S.A. et al. Acceleration of conduction velocity linked to clustering of nodal components precedes myelination. Proc. Natl. Acad. Sci. USA 112, E321–E328 (2015).
Fünfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Volkmar, F.R. & Greenough, W.T. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science 176, 1445–1447 (1972).
Bliss, T.V. & Collingridge, G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Milner, B., Squire, L.R. & Kandel, E.R. Cognitive neuroscience and the study of memory. Neuron 20, 445–468 (1998).
Lee, K.J., Rhyu, I.J. & Pak, D.T. Synapses need coordination to learn motor skills. Rev. Neurosci. 25, 223–230 (2014).
Xu, T. et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919 (2009).
Li, Q., Brus-Ramer, M., Martin, J.H. & McDonald, J.W. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci. Lett. 479, 128–133 (2010).
Gibson, E.M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
Greiner-Tollersrud, L., Berg, T., Stensland, H.M., Evjen, G. & Greiner-Tollersrud, O.K. Bovine brain myelin glycerophosphocholine choline phosphodiesterase is an alkaline lysosphingomyelinase of the eNPP-family, regulated by lysosomal sorting. Neurochem. Res. 38, 300–310 (2013).
Sakagami, H. et al. Biochemical and molecular characterization of a novel choline-specific glycerophosphodiester phosphodiesterase belonging to the nucleotide pyrophosphatase/phosphodiesterase family. J. Biol. Chem. 280, 23084–23093 (2005).
Morita, J. et al. Structure and biological function of ENPP6, a choline-specific glycerophosphodiester-phosphodiesterase. Sci. Rep. 6, 20995 (2016).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Hughes, E.G., Kang, S.H., Fukaya, M. & Bergles, D.E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 16, 668–676 (2013).
Walker, M.P., Brakefield, T., Morgan, A., Hobson, J.A. & Stickgold, R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35, 205–211 (2002).
Trapp, B.D., Nishiyama, A., Cheng, D. & Macklin, W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J. Cell Biol. 137, 459–468 (1997).
Chang, A., Tourtellotte, W.W., Rudick, R. & Trapp, B.D. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl. J. Med. 346, 165–173 (2002).
Matsumoto, Y. et al. Differential proliferation rhythm of neural progenitor and oligodendrocyte precursor cells in the young adult hippocampus. PLoS One 6, e27628 (2011).
Bellesi, M. Sleep and oligodendrocyte functions. Curr Sleep Med Rep. 1, 20–26 (2015).
van Heyningen, P., Calver, A.R. & Richardson, W.D. Control of progenitor cell number by mitogen supply and demand. Curr. Biol. 11, 232–241 (2001).
Draganski, B. et al. Neuroplasticity: changes in grey matter induced by training. Nature 427, 311–312 (2004).
Scholz, J., Klein, M.C., Behrens, T.E. & Johansen-Berg, H. Training induces changes in white-matter architecture. Nat. Neurosci. 12, 1370–1371 (2009).
Hu, Y. et al. Enhanced white matter tracts integrity in children with abacus training. Hum. Brain Mapp. 32, 10–21 (2011).
Sampaio-Baptista, C. et al. Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci. 33, 19499–19503 (2013).
Sagi, Y. et al. Learning in the fast lane: new insights into neuroplasticity. Neuron 73, 1195–1203 (2012).
Ho, V.M., Lee, J.A. & Martin, K.C. The cell biology of synaptic plasticity. Science 334, 623–628 (2011).
Kole, K. Experience-dependent plasticity of neurovascularization. J. Neurophysiol. 114, 2077–2079 (2015).
Makinodan, M., Rosen, K.M., Ito, S. & Corfas, G. A critical period for experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012).
Mangin, J.M., Li, P., Scafidi, J. & Gallo, V. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat. Neurosci. 15, 1192–1194 (2012).
Bergles, D.E. & Richardson, W.D. Oligodendrocyte development and plasticity. in Glia (eds. Barres, B.A., Freeman, M.R. & Stevens, B.) 139–165 (Cold Spring Harbor, 2015).
Hines, J.H., Ravanelli, A.M., Schwindt, R., Scott, E.K. & Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015).
Mensch, S. et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630 (2015).
Jolly, S., Fudge, A., Pringle, N., Richardson, W.D. & Li, H. Combining double fluorescence in situ hybridization with immunolabelling for detection of the expression of three genes in mouse brain sections. J. Vis. Exp. 109 10.3791/53976 (2016).
Acknowledgements
We thank our colleagues at University College London, especially S. Jolly, U. Grazini and L. Magno, for advice and reagents, and M. Grist and U. Dennehy for technical help. We thank M. Wegner (University of Erlangen, Germany) for the Sox10 antibody. This work was supported by the European Research Council (grant agreement 293544 to W.D.R.), the Wellcome Trust (100269/Z/12/Z to W.D.R.) and the Biotechnology and Biological Sciences Research Council (BB/L003236/1 to H.L.). L.X. was supported by the National Natural Science Foundation of China (grant 31471013). Pdgfra-CreERT2 mice can be obtained through http://www.e-lucid.com/ with a material transfer agreement. Myrf loxP mice are available from Jackson Labs, strain 010607.
Author information
Authors and Affiliations
Contributions
W.D.R. formed the hypotheses and obtained funding. I.A.M. adopted and developed the complex wheel test. B.E. provided MyrfloxP mice, advice and suggestions. W.D.R., I.A.M. and D.O. designed the experiments in Figure 1 and Supplementary Figure 1; D.O. and I.A.M. performed those experiments and DO analyzed the data. W.D.R., H.L. and L.X. designed all the other experiments and L.X. performed them, with assistance from A.S.-W., J.L.W. and A.D.F. H.L. identified Enpp6 and A.F. performed preliminary characterization. H.L. and W.D.R. supervised the work. W.D.R. wrote the paper with input from H.L., L.X. and B.E.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
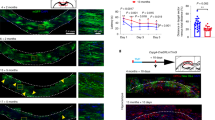
Supplementary Figure 1 Performance of P‑Myrf−/− versus P‑Myrf+/− mice on the complex wheel.
Three weeks after tamoxifen administration at P60-64 or P90-P94 (9), P‑Myrf−/− mice and their P‑Myrf+/− littermates [n=36 (20 males) and 32 (17 males) respectively] were housed singly in cages containing a complex wheel. Average wheel speeds were calculated for each 2-hour time window during the first seven nights (6pm-6am) and plotted as mean ± s.e.m (P‑Myrf−/−, red; P‑Myrf+/−, blue) were analyzed by two-way ANOVA with Bonferroni's post-hoc test. Each night was treated separately for multiple comparisons. *p < 0.05, **p < 0.01, ***p < 10−3, ****p < 10−4
[Night 1: 2 h, p=0.43, t=1.81; 4 h, p=0.029, t=2.83; 6 h, p=0.0089, t=3.20; 8 h, p=0.0044, t=3.40; 10 h, p=0.0017, t=3.66; 12 h, p=0.0036, t=3.46. Night 2: 2 h, p=0.0067, t=3.28; 4 h, p=0.0033, t=3.48; 6 h, p=0.063, t=2.57; 8 h, p=0.016, t=3.03; 10 h, p=0.11, t=2.37; 12 h, p=0.034, t=2.79. Night 3: 2 h, p=0.0019, t=3.63; 4 h, p=0.0041, t=3.43; 6 h, p=0.020, t=2.95; 8 h, p=0.072, t=2.53; 10 h, p>0.99, t=1.05; 12 h, p=0.24, t=2.06. Night 4: 2 h, p=0.020, t=2.96; 4 h, p=0.0010, t=3.80; 6 h, p=0.0007, t=3.90; 8 h, p=0.020, t=2.96; 10 h, p=0.033, t=2.79; 12 h, p=0.30, t=1.96. Night 5: 2 h, p=0.0006, t=3.94; 4 h, p=0.0052, t=3.36; 6 h, p=0.015, t=3.04; 8 h, p=0.13, t=2.32; 10 h, p=0.59, t=1.66; 12 h, p=0.17, t=2.42. Night 6: p<0.0001, t=4.42; 4 h, p<0.0001, t=4.42; 6 h, p<0.0001, t=4.46; 8 h, p=0.0046, t=3.39; 10 h, p=0.020, t=2.96; 12 h, p=0.095, t=2.42. Night 7: 2 h, p<0.0001, t=4.65; 4 h, p<0.0001, t=4.39; 6 h, p=0.0001, t=4.32; 8 h, p=0.0004, t=4.05; 10 h, p=0.20, t=2.13; 12 h, p=0.097, t=2.42. Degrees of freedom=396 throughout.]
Supplementary Figure 2 Two populations of high- and low-expressing Enpp6+ cells in vivo.
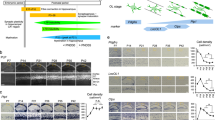
(a,b) Coronal sections of P60 mouse forebrains were incubated with an Enpp6 ISH probe and the signal developed using the NBT/BCIP method (Methods). (a) At a relatively long development time (150 minutes) two populations of Enpp6-positive cells are detected in the subcortical white matter – a few strongly-labeled large cell bodies (arrows) against a background of more numerous, small, weakly-labeled cells (arrowheads), consistent with the RNA-seq data27 (Fig. 3A) and the likelihood that the strongly-labeled cells are newly-differentiating oligodendrocytes and the weakly-labeled cells more mature, myelinating oligodendrocytes. (b) Reducing the development time to 60 minutes allows the strongly-labeled cells to be visualized preferentially (arrows). Images are representative of >3 similar experiments. Scale bar, 50 μm.
Supplementary Figure 3 Enpp6 expression is oligodendrocyte-specific.
Forebrain sections of P90 mice (caged without wheels) were analyzed by ISH for Enpp6 mRNA followed by immuno-fluorescence labeling for stage-specific markers of the oligodendrocyte lineage (Sox10, Olig2, CC1). Alternatively, sections were analyzed by double fluorescence ISH for Enpp6 and Pdgfra. (a,b) All Enpp6+ cells were also Olig2+ and Sox10+. (c) No Enpp6+ cells were Pdgfra+ OPs. (d) Most Enpp6+ cells were CC1+ mature or maturing oligodendrocytes (Motor cortex, 98.2 ± 1.8%; Subcortical white matter, 86.9% ± 3.8%) (Supplementary Table 1). Images are representative of >3 similar experiments. Scale bar, 25 μm.
Supplementary Figure 4 The role of novel motor activity in stimulating OP differentiation.
OPs are continuously cycling in the young adult CNS in response to mitogenic growth factors such Pdgf. After division one or both daughter OPs can rest in the G1 phase of the cell cycle, sometimes for days or weeks, before either differentiating or entering another division cycle3,4,7,9,31. Our data suggest that electrical activity in axon(s) cans stimulate OPs that are paused in G1 to differentiate - losing Pdgfra expression and expressing Enpp6, Mbp and other myelin gene products instead. The newly-differentiating oligodendrocytes have a distinctive spidery morphology in vivo; they remain like this for several days in vivo in rodents31 before down-regulating Enpp6 (faint pink line) and assuming the typical morphology of mature myelinating oligodendrocytes. The Enpp6high early-differentiating oligodendrocytes and Enpp6low myelinating oligodendrocytes probably contribute to improving circuit performance in the early and late stages of motor learning, respectively.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Table 1 (PDF 1064 kb)
Rights and permissions
About this article
Cite this article
Xiao, L., Ohayon, D., McKenzie, I. et al. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci 19, 1210–1217 (2016). https://doi.org/10.1038/nn.4351
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4351
This article is cited by
-
Oligodendrocyte progenitor cells in Alzheimer’s disease: from physiology to pathology
Translational Neurodegeneration (2023)
-
Single-cell RNA-sequencing identifies disease-associated oligodendrocytes in male APP NL-G-F and 5XFAD mice
Nature Communications (2023)
-
Characterization of a new mouse line triggering transient oligodendrocyte progenitor depletion
Scientific Reports (2023)
-
Norepinephrine modulates calcium dynamics in cortical oligodendrocyte precursor cells promoting proliferation during arousal in mice
Nature Neuroscience (2023)
-
Oligodendrocyte dynamics dictate cognitive performance outcomes of working memory training in mice
Nature Communications (2023)