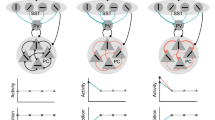
Abstract
Theories have proposed that, in sensory cortices, learning can enhance top-down modulation by higher brain areas while reducing bottom-up sensory drives. To address circuit mechanisms underlying this process, we examined the activity of layer 2/3 (L2/3) excitatory neurons in the mouse primary visual cortex (V1) as well as L4 excitatory neurons, the main bottom-up source, and long-range top-down projections from the retrosplenial cortex (RSC) during associative learning over days using chronic two-photon calcium imaging. During learning, L4 responses gradually weakened, whereas RSC inputs became stronger. Furthermore, L2/3 acquired a ramp-up response temporal profile, potentially encoding the timing of the associated event, which coincided with a similar change in RSC inputs. Learning also reduced the activity of somatostatin-expressing inhibitory neurons (SOM-INs) in V1 that could potentially gate top-down inputs. Finally, RSC inactivation or SOM-IN activation was sufficient to partially reverse the learning-induced changes in L2/3. Together, these results reveal a learning-dependent dynamic shift in the balance between bottom-up and top-down information streams and uncover a role of SOM-INs in controlling this process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
16 July 2015
In the version of this article initially published online, the Acknowledgments listed T.K. as a recipient of the Robertson Stem Cell Prize from the New York Stem Cell Foundation. This should have read a NYSCF-Robertson Investigator. The error has been corrected for the print, PDF and HTML versions of this article.
References
Desimone, R. & Duncan, J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 (1995).
Hupé, J.M. et al. Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature 394, 784–787 (1998).
Engel, A.K., Fries, P. & Singer, W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2, 704–716 (2001).
Krupa, D.J., Wiest, M.C., Shuler, M.G., Laubach, M. & Nicolelis, M.A. Layer-specific somatosensory cortical activation during active tactile discrimination. Science 304, 1989–1992 (2004).
Nienborg, H. & Cumming, B.G. Decision-related activity in sensory neurons reflects more than a neuron's causal effect. Nature 459, 89–92 (2009).
Gilbert, C.D. & Li, W. Top-down influences on visual processing. Nat. Rev. Neurosci. 14, 350–363 (2013).
Harris, K.D. & Mrsic-Flogel, T.D. Cortical connectivity and sensory coding. Nature 503, 51–58 (2013).
Rao, R.P. & Ballard, D.H. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2, 79–87 (1999).
Hinton, G.E. Learning multiple layers of representation. Trends Cogn. Sci. 11, 428–434 (2007).
Friston, K. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 11, 127–138 (2010).
Olshausen, B.A. & Field, D.J. Sparse coding of sensory inputs. Curr. Opin. Neurobiol. 14, 481–487 (2004).
Bastos, A.M. et al. Canonical microcircuits for predictive coding. Neuron 76, 695–711 (2012).
Gdalyahu, A. et al. Associative fear learning enhances sparse network coding in primary sensory cortex. Neuron 75, 121–132 (2012).
Mignard, M. & Malpeli, J.G. Paths of information flow through visual cortex. Science 251, 1249–1251 (1991).
Felleman, D.J. & Van Essen, D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991).
Petreanu, L., Mao, T., Sternson, S.M. & Svoboda, K. The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145 (2009).
Cauller, L.J. & Connors, B.W. Synaptic physiology of horizontal afferents to layer I in slices of rat SI neocortex. J. Neurosci. 14, 751–762 (1994).
Zhang, S. et al. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345, 660–665 (2014).
Vann, S.D., Aggleton, J.P. & Maguire, E.A. What does the retrosplenial cortex do? Nat. Rev. Neurosci. 10, 792–802 (2009).
Lukoyanov, N.V. & Lukoyanova, E.A. Retrosplenial cortex lesions impair acquisition of active avoidance while sparing fear-based emotional memory. Behav. Brain Res. 173, 229–236 (2006).
Gentet, L.J. et al. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat. Neurosci. 15, 607–612 (2012).
Palmer, L., Murayama, M. & Larkum, M. Inhibitory regulation of dendritic activity in vivo. Front. Neural Circuits 6, 26 (2012).
Dombeck, D.A., Harvey, C.D., Tian, L., Looger, L.L. & Tank, D.W. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 13, 1433–1440 (2010).
Glickfeld, L.L., Histed, M.H. & Maunsell, J.H. Mouse primary visual cortex is used to detect both orientation and contrast changes. J. Neurosci. 33, 19416–19422 (2013).
Chen, T.W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Peters, A.J., Chen, S.X. & Komiyama, T. Emergence of reproducible spatiotemporal activity during motor learning. Nature 510, 263–267 (2014).
Janssen, P. & Shadlen, M.N. A representation of the hazard rate of elapsed time in macaque area LIP. Nat. Neurosci. 8, 234–241 (2005).
Shuler, M.G. & Bear, M.F. Reward timing in the primary visual cortex. Science 311, 1606–1609 (2006).
Niell, C.M. & Stryker, M.P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010).
Cauller, L. Layer I of primary sensory neocortex: where top-down converges upon bottom-up. Behav. Brain Res. 71, 163–170 (1995).
Kawaguchi, Y. & Kubota, Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 7, 476–486 (1997).
Markram, H. et al. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807 (2004).
Brown, S.P. & Hestrin, S. Cell-type identity: a key to unlocking the function of neocortical circuits. Curr. Opin. Neurobiol. 19, 415–421 (2009).
Kepecs, A. & Fishell, G. Interneuron cell types are fit to function. Nature 505, 318–326 (2014).
Lovett-Barron, M. et al. Dendritic inhibition in the hippocampus supports fear learning. Science 343, 857–863 (2014).
Ma, W.P. et al. Visual representations by cortical somatostatin inhibitory neurons: selective but with weak and delayed responses. J. Neurosci. 30, 14371–14379 (2010).
Kerlin, A.M., Andermann, M.L., Berezovskii, V.K. & Reid, R.C. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67, 858–871 (2010).
Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Callaway, E.M. Feedforward, feedback and inhibitory connections in primate visual cortex. Neural Netw. 17, 625–632 (2004).
Vélez-Fort, M. et al. The stimulus selectivity and connectivity of layer six principal cells reveals cortical microcircuits underlying visual processing. Neuron 83, 1431–1443 (2014).
Olsen, S.R., Bortone, D.S., Adesnik, H. & Scanziani, M. Gain control by layer six in cortical circuits of vision. Nature 483, 47–52 (2012).
Bortone, D.S., Olsen, S.R. & Scanziani, M. Translaminar inhibitory cells recruited by layer 6 corticothalamic neurons suppress visual cortex. Neuron 82, 474–485 (2014).
Cottam, J.C., Smith, S.L. & Hausser, M. Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J. Neurosci. 33, 19567–19578 (2013).
Pfeffer, C.K., Xue, M., He, M., Huang, Z.J. & Scanziani, M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 16, 1068–1076 (2013).
Xu, H., Jeong, H.Y., Tremblay, R. & Rudy, B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron 77, 155–167 (2013).
Letzkus, J.J. et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335 (2011).
Deco, G. & Rolls, E.T. A neurodynamical cortical model of visual attention and invariant object recognition. Vision Res. 44, 621–642 (2004).
Larkum, M. A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 36, 141–151 (2013).
Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 10, 433–436 (1997).
Pologruto, T.A., Sabatini, B.L. & Svoboda, K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003).
Thévenaz, P., Ruttimann, U.E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 (1998).
Dombeck, D.A., Khabbaz, A.N., Collman, F., Adelman, T.L. & Tank, D.W. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56, 43–57 (2007).
Ringach, D.L., Hawken, M.J. & Shapley, R. Dynamics of orientation tuning in macaque primary visual cortex. Nature 387, 281–284 (1997).
Paxinos, G. & Franklin, K.B.J. Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates (Boston: Elsevier/Academic Press, Amsterdam, 2013).
Zhao, S. et al. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat. Methods 8, 745–752 (2011).
Acknowledgements
We thank A. Kim and L. Xiao for technical assistance, L.L. Looger, J. Akerboom, D.S. Kim and the GENIE Project at Janelia Farm for making GCaMP available, S. Olsen, B. Liu, S. Ruediger-Lee and M. Scanziani for help with visual stimulation and circular treadmill, S. Shabel and R. Malinow for help with slice experiments, and M. Basso, R. Malinow, J. Serences and members of the Komiyama laboratory for comments and discussions. This work was supported by grants from the US National Institutes of Health (1R01NS091010-01, 1R01DC014690-01), Japan Science and Technology Agency (PRESTO), Pew Charitable Trusts, Alfred P. Sloan Foundation, David & Lucile Packard Foundation, Human Frontier Science Program, McKnight Foundation, and the New York Stem Cell Foundation (NYSCF) to T.K. H.M. was supported by the Uehara Memorial Foundation Research Fellowship and the JSPS postdoctoral fellowship for Research Abroad. T.K. is a NYSCF-Robertson Investigator.
Author information
Authors and Affiliations
Contributions
H.M. and T.K. conceived the project. H.M. performed the experiments. H.M. and T.K. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Tuning properties of individual circuit components and stimulus-specificity of experience-driven changes.
(a) Left, circuit schematic with the imaged component (L2/3 excitatory neurons) shown in green and examples of their tuning properties. Right, histograms of the orientation selectivity index (OSI) and direction selectivity index (DSI) of L2/3 excitatory neurons (n = 448 neurons, 13 mice). (b) Changes in tuning properties of L2/3 excitatory neurons after passive experience or learning (passive: 6 mice; learning: 5 mice). In a subset of mice, tuning properties of each circuit component were measured again on day 5 and these values were compared to those of day 0. Population response change was defined as in Figure 3. T indicates the target stimulus. (c-d) Data are presented as in a-b for RSC axonal boutons (c, n = 599 boutons, 12 mice; d, passive: 6 mice; learning: 6 mice). (e-f) Data are presented as in a-b for L4 excitatory neurons (e, n = 378 neurons, 10 mice; f, passive: 5 mice; learning: 5 mice). (g-h) Data are presented as in a-b for SOM-INs (g, n = 100 neurons, 14 mice; h, passive: 7 mice; learning: 7 mice). (i-j) Data are presented as in a-b for PV-INs (i, n = 127 neurons, 11 mice; j, passive: 6 mice; learning: 5 mice). (k-l) Date are presented as in a-b for VIP-INs (k, n = 123 neurons, 9 mice; l, passive: 5 mice; learning: 4 mice). (m) Distribution of the target stimuli used in this study (passive: 35 mice; learning: 67 mice).
Supplementary Figure 2 Imaging GCaMP6f across cortical layers.
(a) Coronal slice of V1 expressing GCaMP6f, with tdTomato marking inhibitory neurons. GCaMP6f expression is evident in all layers including L4 which shows weaker expression than other layers. (b) In vivo images of different layers. L5 was readily identifiable by the appearance of large cell bodies (arrowheads) and avoided during L4 imaging (n = 3 mice).
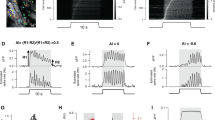
Supplementary Figure 3 Experience-dependent changes in response properties of each excitatory circuit component.
(a) Circuit schematic with the imaged component (RSC axonal boutons) shown in green. (b) Top left, heat maps of normalized trial-average dF/F of individual RSC axonal boutons responsive at either time point and their mean trial-average dF/F. Boutons are sorted in the order of peak timing of the naive condition in both cases so that direct cell-to-cell comparisons can be made. Black boxes indicate non-responsive boutons either because they stopped responding after experience or they were non-responsive in the naive state but became responsive after experience. Bottom left, example responses of boutons. Middle, naive and learning conditions. Right, learning and post-learning anesthesia conditions. (c-d) Data are presented as in a-b for L2/3 excitatory neurons. (e-f) Data are presented as in a-b for L4 excitatory neurons. (g) RSC inactivation in the naive state does not change the ramp index of L2/3 excitatory neurons (P = 0.43, one-tailed bootstrap, control: n = 146 neurons, 13 mice (adapted from Figure 4h); RSC inactivation: n = 11 neurons, 3 mice).
Supplementary Figure 4 Ramp index over sessions in each excitatory circuit component.
(a) Circuit schematic with the imaged component (RSC axonal boutons) shown in green. (b) Top, mean ramp index of responsive RSC axonal boutons (P = 0.12, one-way ANOVA, n = 6 mice, left) and mean trial average dF/F of the RSC axonal boutons that were responsive at each time point across sessions (right) for the passive condition. Bottom, learning condition (*P = 0.019, n = 6 mice). Inset, population activity in blocks of 5 trials each from the earlier phase of day 1. (c) Behavioral performance of the RSC learning group. (d-f) Data are presented as in a-c for L2/3 excitatory neurons (e, passive: P = 0.39, n = 5 mice; learning: ***P < 0.001, n = 7 mice). (g-i) Data are presented as in a-c for L4 excitatory neurons (h, passive: P = 0.60, n = 5 mice; learning: P = 0.98, n = 5 mice).
Supplementary Figure 5 Running does not affect the post-learning response of L2/3 excitatory neurons.
(a) Left, circuit schematic with the imaged component (L2/3 excitatory neurons) shown in green. Right, responses of four example L2/3 excitatory neurons in hit (left) and miss (right) trials in the last two sessions (days 3 and 4). Thin line, individual trials; thick lines, average. Individual hit trials for this panel were selected from a trial before and after each miss trial. In all miss trials included in this figure, mice did not run above threshold during the response period. Note that L2/3 excitatory neurons showed ramp-up activity in both hit and miss trials. (b) Top, mean trial-average dF/F in hit and miss trials, averaged across all responsive L2/3 excitatory neurons, showing indistinguishable responses in both trial types. The miss trial trace appears noisier due to the fewer number of trials. Bottom, mean running traces in hit and miss trials. (c) Running enhances L2/3 excitatory neuron responses in naive (***P < 0.001, Wilcoxon signed-rank test, n = 167 neurons, 12 mice) and passive (***P < 0.001, n = 37 neurons, 6 mice), but not in learning conditions (P = 0.34, n = 70 neurons, 7 mice). Stationary and running trials were the trials in which peak running speed was below and above the threshold, respectively. These correspond to miss and hit trials in the learning condition.
Supplementary Figure 6 Visual stimulus-shock association (‘conditioning’) induces ramp-up responses in RSC axons and L2/3 excitatory neurons independent of running.
(a) Schematic of the experiment. Tail shock was given in randomly-selected 80% of the trials and responses were analyzed in the remaining 20% of the trials without the shock. (b) Mean ramp index for RSC axonal boutons (*P = 0.017, one-tailed bootstrap, naive: n = 22 boutons, 3 mice; conditioning: n = 66 boutons, 3 mice) and L2/3 excitatory neurons (**P = 0.001, naive: n = 38 neurons, 7 mice; conditioning: n = 16 neurons, 7 mice). (c) Left, circuit schematic with the imaged component (RSC axonal boutons) shown in green. Top right, heat maps of normalized trial-average dF/F of individual boutons sorted by peak timing. Middle right, mean dF/F of all responsive boutons within each condition. Bottom right, mean running traces in each group, showing that mice generally did not run in this paradigm. (d) Data are presented as in c for L2/3 excitatory neurons.
Supplementary Figure 7 Mean and example responses of SOM-INs.
(a) Left, circuit schematic with the imaged component (SOM-INs) shown in green. Middle, mean trial-average dF/F of SOM-INs that are responsive in either of the two compared conditions (naive/passive: n = 41 neurons, 7 mice; naive/learning: n = 29 neurons, 7 mice; learning/post-learning anesthesia: n = 6 neurons, 2 mice). Right, example responses of SOM-INs. (b) Left, circuit schematic with the imaged component (SOM-INs) shown in green. SOM-IN activity was imaged after learning while RSC was inactivated with muscimol injections. Right, mean trial-average dF/F of SOM-INs that are responsive in at least one of the three conditions. RSC inactivation after learning suppresses SOM-IN activity (n = 8 neurons, 2 mice).
Supplementary Figure 8 Response dynamics of parvalbumin (PV) and vasoactive intestinal peptide (VIP) expressing inhibitory neurons during passive experience and learning.
(a) Left, circuit schematic with the imaged component (PV-INs) shown in green. Right, same population of PV-INs expressing GCaMP6f imaged 4 d apart. (b) Spatial map of responsive PV-INs in an image field from a mouse before (left) and after passive experience or learning (right), pseudocolor-coded according to the activity levels. (c) Left, population response change of PV-INs over days in passive and learning groups (passive: p < 0.001, n = 31 neurons, 6 mice; learning: p < 0.001, n = 34 neurons, 5 mice, one-way repeated measures ANOVA). Middle, changes in the number of responsive PV-INs at each time point normalized to the value on day 0 in passive and learning groups (passive: p < 0.001, n = 6 mice; learning: p < 0.001, n = 5 mice, one-way repeated measures ANOVA). Right, dF/F of the PV-INs that were responsive at each time point in passive and learning groups. n.d. = not determined because there were no responsive neurons. (d-f) Data are presented as in a-c for VIP-INs. Left in f: passive: p < 0.001, n = 28 neurons, 5 mice; learning: p < 0.001, n = 31 neurons, 4 mice. Middle in f: passive: p < 0.001, n = 5 mice; learning: p < 0.001, n = 4 mice. (g) Example activity of all VIP-INs imaged from one mouse during passive experience (left) or learning (right). Note that in both groups running increases spontaneous activity of VIP-INs but visual stimuli suppress such running-induced activity, indicated by arrows. (h) Top, mean visually-evoked dF/F of all VIP-INs in running or stationary trials for the passive (left) and learning (right) groups. Bottom, mean spontaneous or evoked dF/F in each condition (passive: ***P < 0.001, n = 117 neurons, 5 mice, Wilcoxon signed-rank test with Bonferroni correction; learning: ***P < 0.001, n = 105 neurons, 4 mice).
Supplementary Figure 9 Validation of SSFO.
(a) Schematic of the experiment. Whole-cell current-clamp recordings were performed in SOM-INs expressing SSFO-EYFP in V1 acute slices. (b) Example SOM-IN showing changes in the membrane potential by SSFO activation with 470 nm blue light and deactivation with 590 nm amber light. (c) Changes in the mean membrane potential of SOM-INs (***P < 0.001, P > 0.05 between naive and deactivation, one-way repeated measures ANOVA with post-hoc Tukey test, n = 5 neurons, 3 mice). (d) Example SOM-IN showing excitability changes by SSFO activation and deactivation with current injections. (e) Changes in firing rates by SSFO activation (left) and deactivation (right) with different current injections. Different symbols indicate different cells. (f) Left, L2/3 PV-INs expressing SSFO-EYFP (arrowheads) and putative excitatory neurons expressing GCaMP6f imaged in vivo. Right, model of the experiment. (g) Left, visually-evoked activity of three example L2/3 putative excitatory neurons before and after SSFO activation and deactivation in PV-INs. Right, mean dF/F of L2/3 putative excitatory neurons by SSFO activation and deactivation in PV-INs (***P < 0.001, **P = 0.001, P > 0.05 between naive and deactivation, Wilcoxon signed-rank test with Bonferroni correction, n = 47 neurons, 3 mice). (h) Control for the SOM-IN reactivation experiment (Figure 6d-g) without SSFO expression. Left, schematic of the experiment. Middle, changes in the ramp index of individual L2/3 excitatory neurons by blue light (P = 0.23, Wilcoxon signed-rank test, n = 26 neurons, 5 mice). Right, changes in the ramp index of the same neurons by amber light (P = 0.32, n = 26 neurons, 5 mice). The pie chart illustrates the fractions of neurons showing a significant increase or decrease in the ramp index. (i) Model of SOM-IN activation after learning. SOM-INs gate top-down inputs arriving at distal dendrites. SOM-INs may also inhibit another class of inhibitory neurons (‘IN’) that normally gate bottom-up inputs arriving at the perisomatic dendrites of L2/3 excitatory neurons. After learning, these INs are released from SOM-IN inhibition and become active to gate off the bottom-up inputs. SOM-IN activation suppresses these INs and disinhibits the bottom-up inputs.
Supplementary Figure 10 Model.
Top, summary of our findings. Passive experience and associative learning shift the relative balance in the input strengths between the top-down pathway from RSC and bottom-up pathway from L4 to L2/3. In addition, the activity change of SOM-INs alters the way the two distinct inputs are processed. In the naive condition (left), SOM-INs are relatively active, gating off top-down inputs arriving at L1. After learning (right), SOM-INs become less active, allowing the top-down inputs to have a stronger impact on L2/3 excitatory neurons. Bottom, hypothetical circuits underlying our findings. RSC provides top-down excitatory inputs to the distal dendrites of L2/3 excitatory neurons and indirectly inhibits the bottom-up inputs through L6 NTSR1 neurons (NRT: thalamic reticular nucleus; dLGN: dorsal lateral geniculate nucleus). SOM-INs inhibit the distal dendrites of L2/3 neurons receiving top-down inputs and indirectly disinhibit basal dendrites receiving bottom-up inputs. These mechanisms allow a dynamic and analog control of the relative balance between bottom-up and top-down processing.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1953 kb)
Rights and permissions
About this article
Cite this article
Makino, H., Komiyama, T. Learning enhances the relative impact of top-down processing in the visual cortex. Nat Neurosci 18, 1116–1122 (2015). https://doi.org/10.1038/nn.4061
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4061
This article is cited by
-
An adaptive behavioral control motif mediated by cortical axo-axonic inhibition
Nature Neuroscience (2023)
-
A frontal transcallosal inhibition loop mediates interhemispheric balance in visuospatial processing
Nature Communications (2023)
-
Contextual Fear Learning and Extinction in the Primary Visual Cortex of Mice
Neuroscience Bulletin (2023)
-
Learning enhances encoding of time and temporal surprise in mouse primary sensory cortex
Nature Communications (2022)
-
Acquiring new memories in neocortex of hippocampal-lesioned mice
Nature Communications (2022)