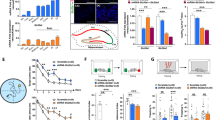
Abstract
In Noonan syndrome (NS) 30–50% of subjects show cognitive deficits of unknown etiology and with no known treatment. Here, we report that knock-in mice expressing either of two NS-associated mutations in Ptpn11, which encodes the nonreceptor protein tyrosine phosphatase Shp2, show hippocampal-dependent impairments in spatial learning and deficits in hippocampal long-term potentiation (LTP). In addition, viral overexpression of an NS-associated allele PTPN11D61G in adult mouse hippocampus results in increased baseline excitatory synaptic function and deficits in LTP and spatial learning, which can be reversed by a mitogen-activated protein kinase kinase (MEK) inhibitor. Furthermore, brief treatment with lovastatin reduces activation of the GTPase Ras–extracellular signal-related kinase (Erk) pathway in the brain and normalizes deficits in LTP and learning in adult Ptpn11D61G/+ mice. Our results demonstrate that increased basal Erk activity and corresponding baseline increases in excitatory synaptic function are responsible for the LTP impairments and, consequently, the learning deficits in mouse models of NS. These data also suggest that lovastatin or MEK inhibitors may be useful for treating the cognitive deficits in NS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tartaglia, M. & Gelb, B.D. Noonan syndrome and related disorders: genetics and pathogenesis. Annu. Rev. Genomics Hum. Genet. 6, 45–68 (2005).
Romano, A.A. et al. Noonan syndrome: clinical features, diagnosis, and management guidelines. Pediatrics 126, 746–759 (2010).
Lee, D.A., Portnoy, S., Hill, P., Gillberg, C. & Patton, M.A. Psychological profile of children with Noonan syndrome. Dev. Med. Child Neurol. 47, 35–38 (2005).
van der Burgt, I. et al. Patterns of cognitive functioning in school-aged children with Noonan syndrome associated with variability in phenotypic expression. J. Pediatr. 135, 707–713 (1999).
Cesarini, L. et al. Cognitive profile of disorders associated with dysregulation of the RAS/MAPK signaling cascade. Am. J. Med. Genet. A. 149A, 140–146 (2009).
Pierpont, E.I. et al. Genotype differences in cognitive functioning in Noonan syndrome. Genes Brain Behav. 8, 275–282 (2009).
Verhoeven, W., Wingbermuhle, E., Egger, J., Van der Burgt, I. & Tuinier, S. Noonan syndrome: psychological and psychiatric aspects. Am. J. Med. Genet. A. 146A, 191–196 (2008).
Alfieri, P. et al. Long term memory profile of disorders associated with dysregulation of the RAS-MAPK signaling cascade. Behav. Genet. 41, 423–429 (2011).
Pierpont, E.I., Tworog-Dube, E. & Roberts, A.E. Learning and memory in children with Noonan syndrome. Am. J. Med. Genet. A 161, 2250–2257 (2013).
Zenker, M. Clinical manifestations of mutations in RAS and related intracellular signal transduction factors. Curr. Opin. Pediatr. 23, 443–451 (2011).
Neel, B.G., Gu, H. & Pao, L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28, 284–293 (2003).
Sweatt, J.D. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem. 76, 1–10 (2001).
Fragale, A., Tartaglia, M., Wu, J. & Gelb, B.D. Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation. Hum. Mutat. 23, 267–277 (2004).
Araki, T. et al. Mouse model of Noonan syndrome reveals cell type– and gene dosage–dependent effects of Ptpn11 mutation. Nat. Med. 10, 849–857 (2004).
Keilhack, H., David, F.S., McGregor, M., Cantley, L.C. & Neel, B.G. Diverse biochemical properties of Shp2 mutants. Implications for disease phenotypes. J. Biol. Chem. 280, 30984–30993 (2005).
Araki, T. et al. Noonan syndrome cardiac defects are caused by PTPN11 acting in endocardium to enhance endocardial-mesenchymal transformation. Proc. Natl. Acad. Sci. USA 106, 4736–4741 (2009).
Morris, R.G., Garrud, P., Rawlins, J.N. & O'Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683 (1982).
Tartaglia, M. et al. Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am. J. Hum. Genet. 78, 279–290 (2006).
Lee, Y.S. & Silva, A.J. The molecular and cellular biology of enhanced cognition. Nat. Rev. Neurosci. 10, 126–140 (2009).
Gauthier, A.S. et al. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron 54, 245–262 (2007).
Zhu, J.J., Qin, Y., Zhao, M., Van Aelst, L. & Malinow, R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110, 443–455 (2002).
Li, W. et al. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr. Biol. 15, 1961–1967 (2005).
Sebti, S.M., Tkalcevic, G.T. & Jani, J.P. Lovastatin, a cholesterol biosynthesis inhibitor, inhibits the growth of human H-ras oncogene transformed cells in nude mice. Cancer Commun. 3, 141–147 (1991).
Mailman, T., Hariharan, M. & Karten, B. Inhibition of neuronal cholesterol biosynthesis with lovastatin leads to impaired synaptic vesicle release even in the presence of lipoproteins or geranylgeraniol. J. Neurochem. 119, 1002–1015 (2011).
Lee, S.H. et al. Synapses are regulated by the cytoplasmic tyrosine kinase Fer in a pathway mediated by p120catenin, Fer, SHP-2, and β-catenin. J. Cell Biol. 183, 893–908 (2008).
Pagani, M.R., Oishi, K., Gelb, B.D. & Zhong, Y. The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell 139, 186–198 (2009).
Rumbaugh, G., Adams, J.P., Kim, J.H. & Huganir, R.L. SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc. Natl. Acad. Sci. USA 103, 4344–4351 (2006).
Stornetta, R.L. & Zhu, J.J. Ras and Rap signaling in synaptic plasticity and mental disorders. Neuroscientist 17, 54–78 (2011).
Tidyman, W.E. & Rauen, K.A. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr. Opin. Genet. Dev. 19, 230–236 (2009).
Shilyansky, C., Lee, Y.S. & Silva, A.J. Molecular and cellular mechanisms of learning disabilities: a focus on NF1. Annu. Rev. Neurosci. 33, 221–243 (2010).
Shilyansky, C. et al. Neurofibromin regulates corticostriatal inhibitory networks during working memory performance. Proc. Natl. Acad. Sci. USA 107, 13141–13146 (2010).
Cui, Y. et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell 135, 549–560 (2008).
Costa, R.M. et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415, 526–530 (2002).
Zolotukhin, S. et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28, 158–167 (2002).
Hajos, N., Nusser, Z., Rancz, E.A., Freund, T.F. & Mody, I. Cell type- and synapse-specific variability in synaptic GABAA receptor occupancy. Eur. J. Neurosci. 12, 810–818 (2000).
Acknowledgements
The authors would like to thank I. Mody, T. O'Dell, P. Golshani and members of A.J.S.'s lab for their comments on the manuscript and for valuable discussions; R. Jones and Y. Zhou for helping with electrophysiological analysis; D.Y. Cai for statistical advice; and A. Amin, H. Shan and R. Knier for technical support. This work was supported by MH084315 to A.J.S., NRF-2013R1A1A1006766 and NRF-2013R1A3A1072570 to Y.-S.L, R37 CA49132 to B.G.N and MEST-2012-0005751 to H.K.K. B.G.N. is also a Canada Research Chair, Tier 1, and work in his lab is partially supported by the Ontario Ministry of Health and Long Term Care and the Princess Margaret Cancer Foundation.
Author information
Authors and Affiliations
Contributions
Y.-S.L., D.E. and A.J.S. conceptualized the research, designed the experiments and wrote the manuscript; Y.-S.L., D.E., M.Z., M.K., H.-H.R., C.K. C.I.N. and Y.C. performed behavioral experiments; Y.-S.L performed whole-cell patch clamp recordings; Y.-S.L., M.Z. and Y.S. performed LTP recording and biochemical analyses; J.-Y.O. and H.K.K. performed immunocytochemistry and biotinylation experiments; T.A. and B.G.N. provided Ptpn11D61G/+ and Ptpn11N308D/+ founders, discussed the results and edited the manuscript; D.B. and C.B. packaged viral vectors; Y. -S.L., D.E., M.Z., J.B., H.K.K. and B.-K.K. analyzed the data and discussed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Basal activity of Ptpn11N308D/+ and Ptpn11D61G/+ mutants.
a. Ptpn11N308D/+ mutants and WT controls show comparable latencies to the target platform in the visible-version of water maze training. Ptpn11N308D/+ mice and WT showed comparable performance in the visible platform version of the water maze. F1, 18 = 0.003, P = 0.954. (b-c) In an open field analysis (20 min duration), Ptpn11N308D/+ mutant mice (n = 16) and WT (n = 22) controls showed comparable speed and travel distance (speed, t-test, P = 0.194; distance, t-test, P = 0.225). d. Ptpn11D61G/+ mutants showed significantly longer latency to the platform during training compared to WT controls in the visible version of water maze. Repeated measures ANOVA with genotype as between-subjects factor, F1, 23 = 32.99, P < 0.0001. (e-f) In an open field analysis (20 min duration), Ptpn11D61G/+ mutant mice (n=10) showed significantly slower speed and less travel distance than WT controls (n = 15). t-test, *** P < 0.0001.
Supplementary Figure 2 Probe trials after extended training.
(a-b) Ptpn11N308D/+ and WT controls show comparable performance in probe trials after extended training. Quadrant occupancy (a) and proximity analysis (b) for the probe trial conducted after 5 days of training shows that there is no significant difference between Ptpn11N308D/+ mutants and WT controls.
(c-d) Ptpn11D61G/+ show spatial memory deficits even with additional training. c. Quadrant occupancy for the probe trial conducted after 7-days of training reveals that Ptpn11D61G/+ mice (n = 10) show no preference for the target quadrant, unlike their WT littermates (n = 15) (F3,36 = 1.824, P = 0.160 and F3,56= 36.04, *** P < 0.0001 for Ptpn11D61G/+ and WT, respectively; one-way ANOVA). In addition, Ptpn11D61G/+ mice also spent significant less time in the target quadrant than WT mice (Ptpn11D61G/+, 33.50 ± 6.27 %; WT, 46.79 ± 3.17, * P <0.05; t-test). Pool quadrants; target (T), adjacent right (AR), opposite (O), and adjacent left (AL) quadrant. d. Ptpn11D61G/+ showed significantly longer proximity to the target platform than WT mice in the probe trial given after 7 days training (Ptpn11D61G/+, 48.34 ± 4.11 cm; WT, 38.77 ± 2.01 cm, * P <0.05; t-test).
Supplementary Figure 3 Ptpn11N308D/+ mutants show deficits in contextual fear conditioning.
Mice were trained with two shocks (0.5 mA, 2 s, 1 h interval) for two days and contextual fear memory was assessed for 3 min in the training chamber on the 3rd day. Freezing (%): WT, 58.91 ± 2.50, n = 20; Ptpn11N308D/+, 43.20 ± 6.82, n=15; * P < 0.05; t-test.
Supplementary Figure 4 Basal synaptic transmission and paired-pulse facilitation in NS mice.
a. Basal synaptic transmission was not altered in Ptpn11D61G/+ mice (wild type, n = 9 slices from 7 mice; Ptpn11D61G/+, n = 9 slices from 6 mice; Repeated-measures ANOVA, F1,16 = 0.502, P = 0.489). Plot shows the fEPSP slope as a function of stimulation intensity. b. Presynaptic fiber volley sizes were not different between WT and Ptpn11D61G/+ mice (Repeated-measures ANOVA, F1, 16 = 0.104, P = 0.751). Plot shows the fiber volley size as a function of stimulation intensity. c. Paired-pulse facilitation was not changed in Ptpn11D61G/+ mice (Repeated-measures ANOVA, F1,15 = 0.183, P = 0.674) d. Basal synaptic transmission was normal in Ptpn11N308D/+ mice (wild type, n = 13 slices from 7 mice; Ptpn11N308D/+, n = 11 slices from 6 mice; Repeated-measures ANOVA, F1,22 = 0.194, P = 0.664). e. Presynaptic fiber volley sizes were not different between WT and Ptpn11N308D/+ mice (Repeated-measures ANOVA, F1,22 = 0.067, P = 0.798). Plot shows the fiber volley size as a function of stimulation intensity. f. Paired-pulse facilitation was normal in Ptpn11N308D/+ mice for different inter-stimulus intervals (wild type, n = 8 slices from 6 mice; Ptpn11N308D/+, n = 9 slices from 5 mice; Repeated-measures ANOVA, F1, 15 = 0.0146, P = 0.905).
Supplementary Figure 5 Ptpn11N308D/+ mutants show LTP deficits with a 2-burst TBS induction protocol.
LTP induced by a 2 TBS protocol was significantly reduced in the hippocampal slices from Ptpn11N308D/+ mice compared with their WT littermates (WT, n = 10 slices from 7 mice; Ptpn11N308D/+, n=11 slices from 6 mice; Repeated-measures ANOVA: F1, 19 = 7.448, P < 0.05; last 10 min of recording, WT, 131.3 ± 3.36 %, Ptpn11N308D/+, 117.0 ± 2.02 %, t-test, P < 0.01). fEPSP slopes normalized to the average baseline response before LTP induction (at time 0) are plotted in 2-min blocks. Sample traces show responses during baseline (gray) and the last 10 min (black) of the recording (average of ten traces). Scale: vertical bar, 0.5 mV; horizontal bar, 4 ms. Error bars represent s.e.m.
Supplementary Figure 6 Viral overexpression of AAV-PTPN11D61G.
a. Western blot analyses confirmed the overexpression of SHP2 (255.6 ± 27.69 % in PTPN11D61G-expressing hippocampus compared to GFP-expressing hippocampus, n = 5 per group, P < 0.001). b. PTPN11D61G-expressing slice was stained with SHP2 antibody together with Gad67 antibody as an inhibitory neuronal marker. Most of the SHP2 staining (red) did not overlap with Gad67 (green).
Supplementary Figure 7 Effect of PTPN11D61G overexpression and SL327 treatment on behavior and basal synaptic transmission.
(a-b) Effects on the acquisition of water maze or swimming speed. a. For the latency to the platform during training, repeated-measures ANOVA revealed no difference among the groups (F3, 34 = 0.618, P = 0.608). b. Neither mutant PTPN11 overexpression nor SL327 treatment affect swimming speed in the probe trial (effect of virus, F(1,37)= 0.054, P = 0.818; effect of treatment, F1,37 = 0.240, P = 0.627). (c-d) Basal synaptic transmission and paired-pulse facilitation in PTPN11D61G overexpressing slices. c. Overexpression of PTPN11D61G or SL327 treatment did not affect the basal synaptic transmission in CA3-CA1 synapses. Repeated-measures ANOVA, F3,36 = 0.175, P = 0.912. d. Paired-pulse facilitation was not affected by either PTPN11D61G overexpression or SL327 treatment. Repeated-measures ANOVA, F3,35 = 0.356, P = 0.785.
Supplementary Figure 8 WT PTPN11 overexpression does not affect either basal p-Erk level or learning and memory in water maze tests.
a. Western blot analysis confirmed the overexpression of SHP2 (711.4 ± 42.2 % in wild type PTPN11-transfected hippocampus compared to GFP-transfected hippocampus, n = 5 per group, P < 0.001) b. Wild-type AAV-PTPN11 overexpression does not affect basal p-Erk level in the hippocampus. (Normalized p-Erk: PTPN11, 98.44 ± 11.48 %, n = 5; WT, 100.00 ± 7.53 %, n = 4) c. For the latency to the platform during training in the hidden-platform version of Morris water maze, repeated-measures ANOVA revealed no difference between GFP (n = 9) and PTPN11-transfected (n = 12) mice (F1,19 = 1.518, P = 0.233). (d-e) Wild-type PTPN11- and GFP-transfected controls show comparable memory in the probe trial. Quadrant occupancy (d) and proximity analysis (e) shows that there is no significant difference between PTPN11- and GFP-transfected controls.
Supplementary Figure 9 Effects of SL327 treatment on p-Erk levels in the hippocampus.
a. Hippocampi were isolated 1 h after SL327 (0, 3, 10, 30, 40, and 50 mg/kg) injection (n=2 – 6 per dose). p-Erk levels were normalized to the controls (vehicle injected) and fitted using a variable slope model in Graphpad Prism. b. SL327 treatment reverses increased Erk activation in PTPN11D61G- transfected hippocampi. Left, Representative immunoblot showing p-Erk (upper) and total Erk (lower) in PTPN11D61G- transfected and GFP- transfected hippocampi. Right, Bar graph displaying normalized p-Erk levels (mean ± s.e.m.). n=7-8 per group. t-test, * P <0.05. c. p-Erk is not significantly increased in the hippocampus of Ptpn11N308D/+ mice compared to WT. p-Erk level normalized to WT, 86.08 ± 10.36 %. n=5 slices from 5 mice per group. t-test, P = 0.330.
Supplementary Figure 10 SL327 treatment reversed memory deficits in Ptpn11D61G/+ mice in Morris water maze.
Quadrant occupancy analysis for the probe trial after the 5th day of training reveals that Ptpn11D61G/+/veh mice showed no specific preference for the target quadrant (Dunnett's Multiple Comparison Test after one-way ANOVA, P > 0.05 for T vs AL, T vs O). Ptpn11D61G/+/SL327 groups selectively searched in the target quadrant (one-way ANOVA, F3, 31 = 15.03, ***P < 0.001; T vs. all other quadrants, Dunnett's Multiple Comparison Test, ***P < 0.001).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Table 1 (PDF 1213 kb)
Supplementary Methods Checklist
(PDF 2053 kb)
Rights and permissions
About this article
Cite this article
Lee, YS., Ehninger, D., Zhou, M. et al. Mechanism and treatment for learning and memory deficits in mouse models of Noonan syndrome. Nat Neurosci 17, 1736–1743 (2014). https://doi.org/10.1038/nn.3863
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3863
This article is cited by
-
Assessment of the FRET-based Teen sensor to monitor ERK activation changes preceding morphological defects in a RASopathy zebrafish model and phenotypic rescue by MEK inhibitor
Molecular Medicine (2024)
-
Novel effects of Ras-MAPK pathogenic variants on the developing human brain and their link to gene expression and inhibition abilities
Translational Psychiatry (2023)
-
An Assessment of the Therapeutic Landscape for the Treatment of Heart Disease in the RASopathies
Cardiovascular Drugs and Therapy (2023)
-
Novel therapeutic perspectives in Noonan syndrome and RASopathies
European Journal of Pediatrics (2023)
-
Echinacoside exhibits antidepressant-like effects through AMPAR–Akt/ERK–mTOR pathway stimulation and BDNF expression in mice
Chinese Medicine (2022)