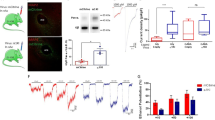
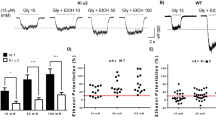
Abstract
The molecular mechanisms that mediate genetic variability in response to alcohol are unclear. We found that alcohol had opposite actions (enhancement or suppression) on GABAA receptor (GABAAR) inhibition in granule cells from the cerebellum of behaviorally sensitive, low alcohol–consuming Sprague-Dawley rats and DBA/2 mice and behaviorally insensitive, high alcohol–consuming C57BL/6 mice, respectively. The effect of alcohol on granule cell GABAAR inhibition was determined by a balance between two opposing effects: enhanced presynaptic vesicular release of GABA via alcohol inhibition of nitric oxide synthase (NOS) and a direct suppression of the activity of postsynaptic GABAARs. The balance of these two processes was determined by differential expression of neuronal NOS (nNOS) and postsynaptic PKC activity, both of which varied across the rodent genotypes. These findings identify opposing molecular processes that differentially control the magnitude and polarity of GABAAR responses to alcohol across rodent genotypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Harwood, H. Updating estimates of the economic costs of alcohol abuse in the United States: estimates, update methods and data. National Institute on Alcohol Abuse and Alcoholism, http://pubs.niaaa.nih.gov/publications/economic-2000/ (2000).
Hill, S.Y. Neural plasticity, human genetics, and risk for alcohol dependence. Int. Rev. Neurobiol. 91, 53–94 (2010).
Prescott, C.A. & Kendler, K.S. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am. J. Psychiatry 156, 34–40 (1999).
Herting, M.M., Fair, D. & Nagel, B.J. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage 54, 2582–2589 (2011).
Schuckit, M.A., Tsuang, J.W., Anthenelli, R.M., Tipp, J.E. & Nurnberger, J.I. Jr. Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. J. Stud. Alcohol 57, 368–377 (1996).
Schuckit, M.A., Smith, T.L., Kalmijn, J. & Danko, G.P. A cross-generational comparison of alcohol challenges at about age 20 in 40 father-offspring pairs. Alcohol. Clin. Exp. Res. 29, 1921–1927 (2005).
Yoneyama, N., Crabbe, J.C., Ford, M.M., Murillo, A. & Finn, D.A. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42, 149–160 (2008).
Gallaher, E.J., Jones, G.E., Belknap, J.K. & Crabbe, J.C. Identification of genetic markers for initial sensitivity and rapid tolerance to ethanol-induced ataxia using quantitative trait locus analysis in BXD recombinant inbred mice. J. Pharmacol. Exp. Ther. 277, 604–612 (1996).
Bell, R.L., et al. Responsivity and development of tolerance to the motor impairing effects of moderate doses of ethanol in alcohol-preferring (P) and -nonpreferring (NP) rat lines. Alcohol. Clin. Exp. Res. 25, 644–650 (2001).
Malila, A. Intoxicating effects of three aliphatic alcohols and barbital on two rat strains genetically selected for their ethanol intake. Pharmacol. Biochem. Behav. 8, 197–201 (1978).
McClearn, G.E., Deitrich, R.A. & Erwin, V.G. Development of Animal Models as Pharmacogentic Tools, Research Monograph No. 6 (National Institute of Alcohol Abuse and Alcoholism, Washington DC, 1981).
Al-Rejaie, S. & Dar, M.S. Antagonism of ethanol ataxia by intracerebellar nicotine: possible modulation by mouse cerebellar nitric oxide and cGMP. Brain Res. Bull. 69, 187–196 (2006).
Duguid, I., Branco, T., London, M., Chadderton, P. & Hausser, M. Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J. Neurosci. 32, 11132–11143 (2012).
Hamann, M., Rossi, D.J. & Attwell, D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron 33, 625–633 (2002).
Brickley, S.G., Revilla, V., Cull-Candy, S.G., Wisden, W. & Farrant, M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409, 88–92 (2001).
Meera, P., Wallner, M. & Otis, T.S. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. J. Neurophysiol. 106, 2057–2064 (2011).
Rossi, D.J., Hamann, M. & Attwell, D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J. Physiol. (Lond.) 548, 97–110 (2003).
Stell, B.M., Brickley, S.G., Tang, C.Y., Farrant, M. & Mody, I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit–containing GABAA receptors. Proc. Natl. Acad. Sci. USA 100, 14439–14444 (2003).
Carta, M., Mameli, M. & Valenzuela, C.F. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J. Neurosci. 24, 3746–3751 (2004).
Hanchar, H.J., Dodson, P.D., Olsen, R.W., Otis, T.S. & Wallner, M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat. Neurosci. 8, 339–345 (2005).
Botta, P. et al. Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiological studies. Alcohol 41, 187–199 (2007).
Melchior, C.L. & Myers, R.D. Genetic differences in ethanol drinking of the rat following injection of 6-OHDA, 5,6-DHT or 5,7-DHT into the cerebral ventricles. Pharmacol. Biochem. Behav. 5, 63–72 (1976).
Korpi, E.R., Kuner, T., Seeburg, P.H. & Luddens, H. Selective antagonist for the cerebellar granule cell-specific gamma-aminobutyric acid type A receptor. Mol. Pharmacol. 47, 283–289 (1995).
Saxena, N.C. & Macdonald, R.L. Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol. Pharmacol. 49, 567–579 (1996).
De Schutter, E. & Bower, J.M. An active membrane model of the cerebellar Purkinje cell II. Simulation of synaptic responses. J. Neurophysiol. 71, 401–419 (1994).
Ford, M.M. et al. The influence of selection for ethanol withdrawal severity on traits associated with ethanol self-administration and reinforcement. Alcohol. Clin. Exp. Res. 35, 326–337 (2011).
Porcu, P. et al. Differential effects of ethanol on serum GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in mice, rats, cynomolgus monkeys, and humans. Alcohol. Clin. Exp. Res. 34, 432–442 (2010).
Botta, P., Simoes de Souza, F.M., Sangrey, T., De Schutter, E. & Valenzuela, C.F. Excitation of rat cerebellar Golgi cells by ethanol: further characterization of the mechanism. Alcohol. Clin. Exp. Res. 36, 616–624 (2012).
Fataccioli, V., Gentil, M., Nordmann, R. & Rouach, H. Inactivation of cerebellar nitric oxide synthase by ethanol in vitro. Alcohol Alcohol. 32, 683–691 (1997).
Wall, M.J. Endogenous nitric oxide modulates GABAergic transmission to granule cells in adult rat cerebellum. Eur. J. Neurosci. 18, 869–878 (2003).
Harris, R.A. et al. Mutant mice lacking the gamma isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of gamma-aminobutyrate type A receptors. Proc. Natl. Acad. Sci. USA 92, 3658–3662 (1995).
Naik, M.U. et al. Distribution of protein kinase Mzeta and the complete protein kinase C isoform family in rat brain. J. Comp. Neurol. 426, 243–258 (2000).
Fidler, T.L. et al. Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure. Genes Brain Behav. 10, 264–275 (2011).
McCool, B.A. & Chappell, A.M. Using monosodium glutamate to initiate ethanol self-administration in inbred mouse strains. Addict. Biol. 17, 121–131 (2012).
Jia, F., Chandra, D., Homanics, G.E. & Harrison, N.L. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J. Pharmacol. Exp. Ther. 326, 475–482 (2008).
Liang, J. et al. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J. Neurosci. 26, 1749–1758 (2006).
Nie, Z., Madamba, S.G. & Siggins, G.R. Ethanol enhances gamma-aminobutyric acid responses in a subpopulation of nucleus accumbens neurons: role of metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 293, 654–661 (2000).
Peris, J., Coleman-Hardee, M., Burry, J. & Pecins-Thompson, M. Selective changes in GABAergic transmission in substantia nigra and superior colliculus caused by ethanol and ethanol withdrawal. Alcohol. Clin. Exp. Res. 16, 311–319 (1992).
Theile, J.W., Morikawa, H., Gonzales, R.A. & Morrisett, R.A. Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J. Pharmacol. Exp. Ther. 329, 625–633 (2009).
Criswell, H.E., Ming, Z., Kelm, M.K. & Breese, G.R. Brain regional differences in the effect of ethanol on GABA release from presynaptic terminals. J. Pharmacol. Exp. Ther. 326, 596–603 (2008).
Kumar, S. et al. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl.) 205, 529–564 (2009).
Korpi, E.R. et al. Cerebellar granule cell–specific GABAA receptors attenuate benzodiazepine-induced ataxia: evidence from alpha 6-subunit-deficient mice. Eur. J. Neurosci. 11, 233–240 (1999).
Mihalek, R.M. et al. GABA(A)-receptor delta subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol. Clin. Exp. Res. 25, 1708–1718 (2001).
White, C.N. et al. Opposing effects of coupled and uncoupled NOS activity on the Na+-K+ pump in cardiac myocytes. Am. J. Physiol. Cell Physiol. 294, C572–C578 (2008).
Salapatek, A.M., Wang, Y.F., Mao, Y.K., Mori, M. & Daniel, E.E. Myogenic NOS in canine lower esophageal sphincter: enzyme activation, substrate recycling, and product actions. Am. J. Physiol. 274, C1145–C1157 (1998).
Wang, W., Hebert, S.C. & Giebisch, G. Renal K+ channels: structure and function. Annu. Rev. Physiol. 59, 413–436 (1997).
Johnson, W.D., Howard, R.J., Trudell, J.R. & Harris, R.A. The TM2 6′ position of GABA(A) receptors mediates alcohol inhibition. J. Pharmacol. Exp. Ther. 340, 445–456 (2012).
Mihic, S.J. et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389, 385–389 (1997).
Ueno, S. et al. Tryptophan scanning mutagenesis in TM2 of the GABA(A) receptor alpha subunit: effects on channel gating and regulation by ethanol. Br. J. Pharmacol. 131, 296–302 (2000).
Yamashita, M., Marszalec, W., Yeh, J.Z. & Narahashi, T. Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABA(A) receptors. J. Pharmacol. Exp. Ther. 319, 431–438 (2006).
Kojima, H. et al. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal. Chem. 70, 2446–2453 (1998).
Acknowledgements
We thank J. Crabbe, D. Finn and K. Wiren (Oregon Health and Science University) for discussions about the research and D. Attwell (University College London) for comments on the manuscript. This study was supported by National Institute of Neurological Disorders and Stroke grant R01NS051561, National Institute on Alcohol Abuse and Alcoholism grant R01AA012439, an American Heart Association Grant in Aid, and a Medical Research Foundation of Oregon grant to D.J.R., training grants T32 AA007468 and F31 AA022267 from the National Institute on Alcohol Abuse and Alcoholism, and an Oregon Health and Science University Research Scholars Award to J.S.K., and the Neuroscience Imaging Center at Oregon Health and Science University grant P30NS061800 from the National Institute of Neurological Disorders and Stroke.
Author information
Authors and Affiliations
Contributions
J.S.K. and D.J.R. designed the experiments. J.S.K. performed the electrophysiology experiments and C.M. performed the immunocytochemistry experiments. J.S.K. and D.J.R. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 2353 kb)
Rights and permissions
About this article
Cite this article
Kaplan, J., Mohr, C. & Rossi, D. Opposite actions of alcohol on tonic GABAA receptor currents mediated by nNOS and PKC activity. Nat Neurosci 16, 1783–1793 (2013). https://doi.org/10.1038/nn.3559
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3559
This article is cited by
-
Cerebellar α6GABAA Receptors as a Therapeutic Target for Essential Tremor: Proof-of-Concept Study with Ethanol and Pyrazoloquinolinones
Neurotherapeutics (2023)
-
Mutation in ε-Sarcoglycan Induces a Myoclonus-Dystonia Syndrome-Like Movement Disorder in Mice
Neuroscience Bulletin (2021)
-
Sensitivity to Sevoflurane anesthesia is decreased in mice with a congenital deletion of Guanylyl Cyclase-1 alpha
BMC Anesthesiology (2017)
-
Ethanol-Induced Cerebellar Ataxia: Cellular and Molecular Mechanisms
The Cerebellum (2015)
-
Mini-Review: Effects of Ethanol on GABAA Receptor-Mediated Neurotransmission in the Cerebellar Cortex—Recent Advances
The Cerebellum (2015)