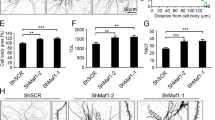
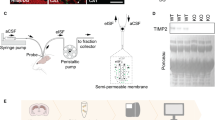
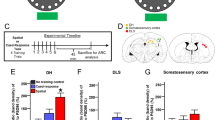
Abstract
Memory formation is thought to be mediated by dendritic-spine growth and restructuring. Myocyte enhancer factor 2 (MEF2) restricts spine growth in vitro, suggesting that this transcription factor negatively regulates the spine remodeling necessary for memory formation. Here we show that memory formation in adult mice was associated with changes in endogenous MEF2 levels and function. Locally and acutely increasing MEF2 function in the dentate gyrus blocked both learning-induced increases in spine density and spatial-memory formation. Increasing MEF2 function in amygdala disrupted fear-memory formation. We rescued MEF2-induced memory disruption by interfering with AMPA receptor endocytosis, suggesting that AMPA receptor trafficking is a key mechanism underlying the effects of MEF2. In contrast, decreasing MEF2 function in dentate gyrus and amygdala facilitated the formation of spatial and fear memory, respectively. These bidirectional effects indicate that MEF2 is a key regulator of plasticity and that relieving the suppressive effects of MEF2-mediated transcription permits memory formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bailey, C.H. & Kandel, E.R. Structural changes accompanying memory storage. Annu. Rev. Physiol. 55, 397–426 (1993).
Harris, K.M. & Stevens, J.K. Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 8, 4455–4469 (1988).
Nimchinsky, E.A., Sabatini, B.L. & Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 64, 313–353 (2002).
Alberini, C.M. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 89, 121–145 (2009).
Abel, T., Martin, K.C., Bartsch, D. & Kandel, E.R. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science 279, 338–341 (1998).
Flavell, S.W. et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 311, 1008–1012 (2006).
Barbosa, A.C. et al. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc. Natl. Acad. Sci. USA 105, 9391–9396 (2008).
Pulipparacharuvil, S. et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron 59, 621–633 (2008).
Lyons, G.E., Micales, B.K., Schwarz, J., Martin, J.F. & Olson, E.N. Expression of mef2 genes in the mouse central nervous system suggests a role in neuronal maturation. J. Neurosci. 15, 5727–5738 (1995).
Flavell, S.W. et al. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron 60, 1022–1038 (2008).
Chowdhury, S. et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron 52, 445–459 (2006).
Shepherd, J.D. et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52, 475–484 (2006).
Morrow, E.M. et al. Identifying autism loci and genes by tracing recent shared ancestry. Science 321, 218–223 (2008).
Greer, P.L. et al. The Angelman syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 140, 704–716 (2010).
Le Meur, N. et al. MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J. Med. Genet. 47, 22–29 (2010).
McNaughton, B.L., Barnes, C.A., Meltzer, J. & Sutherland, R.J. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp. Brain Res. 76, 485–496 (1989).
LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Davis, M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 15, 353–375 (1992).
Fanselow, M.S. & Gale, G.D. The amygdala, fear, and memory. Ann. NY Acad. Sci. 985, 125–134 (2003).
Black, B.L. & Olson, E.N. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 14, 167–196 (1998).
Pfeiffer, B.E. et al. Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron 66, 191–197 (2010).
Chawla, M.K. et al. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus 15, 579–586 (2005).
Frankland, P.W. et al. Consolidation of CS and US representations in associative fear conditioning. Hippocampus 14, 557–569 (2004).
Wiltgen, B.J., Sanders, M.J., Anagnostaras, S.G., Sage, J.R. & Fanselow, M.S. Context fear learning in the absence of the hippocampus. J. Neurosci. 26, 5484–5491 (2006).
Neely, M.D. et al. Localization of myocyte enhancer factor 2 in the rodent forebrain: regionally-specific cytoplasmic expression of MEF2A. Brain Res. 1274, 55–65 (2009).
Sekeres, M.J., Neve, R.L., Frankland, P.W. & Josselyn, S.A. Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learn. Mem. 17, 280–283 (2010).
Josselyn, S.A. et al. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J. Neurosci. 21, 2404–2412 (2001).
McDonald, R.J. & White, N.M. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 107, 3–22 (1993).
Moser, M.B., Trommald, M. & Andersen, P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc. Natl. Acad. Sci. USA 91, 12673–12675 (1994).
O'Malley, A., O'Connell, C. & Regan, C.M. Ultrastructural analysis reveals avoidance conditioning to induce a transient increase in hippocampal dentate spine density in the 6 hour post-training period of consolidation. Neuroscience 87, 607–613 (1998).
Buzsaki, G. The hippocampo-neocortical dialogue. Cereb. Cortex 6, 81–92 (1996).
Wilson, M.A. & McNaughton, B.L. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994).
Shen, J., Kudrimoti, H.S., McNaughton, B.L. & Barnes, C.A. Reactivation of neuronal ensembles in hippocampal dentate gyrus during sleep after spatial experience. J. Sleep Res. 7 (suppl. 1), 6–16 (1998).
Bunch, M.E. & Magdisck, W.K. The retention in rats of an incompletely learned maze solution for short intervals of time. J. Comp. Psychol. 16, 385–409 (1933).
Shepherd, J.D. & Bear, M.F. New views of Arc, a master regulator of synaptic plasticity. Nat. Neurosci. 14, 279–284 (2011).
Rial Verde, E.M., Lee-Osbourne, J., Worley, P.F., Malinow, R. & Cline, H.T. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron 52, 461–474 (2006).
Collingridge, G.L., Isaac, J.T. & Wang, Y.T. Receptor trafficking and synaptic plasticity. Nat. Rev. Neurosci. 5, 952–962 (2004).
Malinow, R. & Malenka, R.C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 (2002).
Gainey, M.A., Hurvitz-Wolff, J.R., Lambo, M.E. & Turrigiano, G.G. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J. Neurosci. 29, 6479–6489 (2009).
Brebner, K. et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 310, 1340–1343 (2005).
Migues, P.V. et al. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat. Neurosci. 13, 630–634 (2010).
Li, H. et al. Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc. Natl. Acad. Sci. USA 105, 9397–9402 (2008).
Cajal, S.R. Sur les ganglions et plexus nerveux de l'intestin. C. R. Soc. Biol. (Paris) 45, 217–223 (1893).
Simon, D.J. et al. The microRNA miR-1 regulates a MEF-2-dependent retrograde signal at neuromuscular junctions. Cell 133, 903–915 (2008).
Pickens, C.L., Golden, S.A., Adams-Deutsch, T., Nair, S.G. & Shaham, Y. Long-lasting incubation of conditioned fear in rats. Biol. Psychiatry 65, 881–886 (2009).
Vetere, G. et al. Spine growth in the anterior cingulate cortex is necessary for the consolidation of contextual fear memory. Proc. Natl. Acad. Sci. USA 108, 8456–8460 (2011).
Kandel, E.R. The biology of memory: a forty-year perspective. J. Neurosci. 29, 12748–12756 (2009).
Ehlers, M.D. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat. Neurosci. 6, 231–242 (2003).
Fioravante, D. & Byrne, J.H. Protein degradation and memory formation. Brain Res. Bull. 85, 14–20 (2011).
Yang, Q. et al. Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science 323, 124–127 (2009).
Keppel, G. & Wickens, T.D. Design and Analysis: A Researcher's Handbook 4th edn. (Prentice Hall, 2004).
Yiu, A.P., Rashid, A.J. & Josselyn, S.A. Increasing CREB function in the CA1 region of dorsal hippocampus rescues the spatial memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology 36, 2169–2186 (2011).
Zalfa, F. et al. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat. Neurosci. 10, 578–587 (2007).
Karamboulas, C. et al. Disruption of MEF2 activity in cardiomyoblasts inhibits cardiomyogenesis. J. Cell Sci. 119, 4315–4321 (2006).
Russo, S.J. et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J. Neurosci. 29, 3529–3537 (2009).
Clark, M.S. et al. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J. Neurosci. 22, 4550–4562 (2002).
Miller, R.R. & McDevitt, C.A. A quantitative microwell assay for chondrocyte cell adhesion. Anal. Biochem. 192, 380–383 (1991).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd edn. (Academic Press, San Diego, 2001).
Teixeira, C.M., Pomedli, S.R., Maei, H.R., Kee, N. & Frankland, P.W. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J. Neurosci. 26, 7555–7564 (2006).
Maei, H.R., Zaslavsky, K., Teixeira, C.M. & Frankland, P.W. What is the most sensitive measure of water maze probe test performance? Front. Integr. Neurosci. 3, 4 (2009).
Acknowledgements
We thank A. DeCristofaro, R. Braybon and M. Yamamoto for excellent technical assistance. This work was supported by grants from the Canadian Institutes of Health Research (CIHR; MOP-74650 (S.A.J.), MOP-86762 (P.W.F.)), EJLB Foundation (S.A.J.) and Natural Science and Engineering Research Council (S.A.J.). C.J.C., A.P.Y. and M.J.S. were supported by Restracomp Fellowships (Hospital for Sick Children), A.P.Y. received support from the Alzheimer's Society of Canada, M.J.S. received a CIHR Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award and a grant from the Faculty of Medicine at the University of Toronto, and C.J.C. received an Ontario Graduate Scholarship.
Author information
Authors and Affiliations
Contributions
S.A.J. and P.W.F. designed, directed and coordinated the study. C.J.C., V.M., L.R. and T.P. conducted behavioral experiments . C.J.C., A.P.Y. and M.J.S. performed the surgeries. C.J.C., V.M. and L.R. performed spine analysis. V.M., L.R. and G.V. performed immunohistochemistry. V.M. and P.J.R. conducted the cell culture experiments. C.J.C., V.M. and L.R. performed statistical analysis. J.-H.H. designed several constructs. R.L.N. generated viral vectors, shRNA and commented extensively on the design of experiments and use of viral vectors. S.A.J. and P.W.F. wrote the manuscript, with assistance from C.J.C., V.M. and L.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 349 kb)
Rights and permissions
About this article
Cite this article
Cole, C., Mercaldo, V., Restivo, L. et al. MEF2 negatively regulates learning-induced structural plasticity and memory formation. Nat Neurosci 15, 1255–1264 (2012). https://doi.org/10.1038/nn.3189
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3189
This article is cited by
-
Early postnatal serotonin modulation prevents adult-stage deficits in Arid1b-deficient mice through synaptic transcriptional reprogramming
Nature Communications (2022)
-
Molecular motor KIF3B in the prelimbic cortex constrains the consolidation of contextual fear memory
Molecular Brain (2021)
-
Synaptic tau: A pathological or physiological phenomenon?
Acta Neuropathologica Communications (2021)
-
A time-dependent role for the transcription factor CREB in neuronal allocation to an engram underlying a fear memory revealed using a novel in vivo optogenetic tool to modulate CREB function
Neuropsychopharmacology (2020)
-
Early-life blockade of NMDA receptors induces epigenetic abnormalities in the adult medial prefrontal cortex: possible involvement in memory impairment in trace fear conditioning
Psychopharmacology (2020)