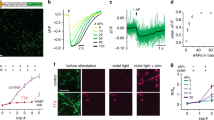
Abstract
The usefulness of genetically encoded probes for optical monitoring of neuronal activity and brain circuits would be greatly advanced by the generation of multiple indicators with non-overlapping color spectra. Most existing indicators are derived from or spectrally convergent on GFP. We generated a bright, red, pH-sensitive fluorescent protein, pHTomato, that can be used in parallel with green probes to monitor neuronal activity. SypHTomato, made by fusing pHTomato to the vesicular membrane protein synaptophysin, reported activity-dependent exocytosis as efficiently as green reporters. When expressed with the GFP-based indicator GCaMP3 in the same neuron, sypHTomato enabled concomitant imaging of transmitter release and presynaptic Ca2+ transients at single nerve terminals. Expressing sypHTomato and GCaMP3 in separate cells enabled the simultaneous determination of presynaptic vesicular turnover and postsynaptic sub- and supra-threshold responses from a connected pair of neurons. With these new tools, we observed a close size matching between pre- and postsynaptic compartments, as well as interesting target cell–dependent regulation of presynaptic vesicle pools. Lastly, by coupling expression of pHTomato- and GFP-based probes with distinct variants of channelrhodopsin, we provided proof-of-principle for an all-optical approach to multiplex control and tracking of distinct circuit pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Siegel, M.S. & Isacoff, E.Y. A genetically encoded optical probe of membrane voltage. Neuron 19, 735–741 (1997).
Mutoh, H., Perron, A., Akemann, W., Iwamoto, Y. & Knopfel, T. Optogenetic monitoring of membrane potentials. Exp. Physiol. 96, 13–18 (2011).
Miyawaki, A. Development of probes for cellular functions using fluorescent proteins and fluorescence resonance energy transfer. Annu. Rev. Biochem. 2011, 357–373 (2011).
Miyawaki, A. et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388, 882–887 (1997).
Kuner, T. & Augustine, G.J. A genetically encoded ratiometric indicator for chloride: capturing chloride transients in cultured hippocampal neurons. Neuron 27, 447–459 (2000).
Miesenböck, G., De Angelis, D.A. & Rothman, J.E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195 (1998).
Miesenböck, G. & Kevrekidis, I.G. Optical imaging and control of genetically designated neurons in functioning circuits. Annu. Rev. Neurosci. 28, 533–563 (2005).
Chow, B.Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 (2010).
Zhang, F. et al. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat. Neurosci. 11, 631–633 (2008).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Shaner, N.C. et al. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat. Methods 5, 545–551 (2008).
Kremers, G.J., Hazelwood, K.L., Murphy, C.S., Davidson, M.W. & Piston, D.W. Photoconversion in orange and red fluorescent proteins. Nat. Methods 6, 355–358 (2009).
Shcherbo, D. et al. Bright far-red fluorescent protein for whole-body imaging. Nat. Methods 4, 741–746 (2007).
Sankaranarayanan, S., De Angelis, D., Rothman, J.E. & Ryan, T.A. The use of pHluorins for optical measurements of presynaptic activity. Biophys. J. 79, 2199–2208 (2000).
Campbell, R.E. et al. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 7877–7882 (2002).
Shaner, N.C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004).
Jach, G., Pesch, M., Richter, K., Frings, S. & Uhrig, J.F. An improved mRFP1 adds red to bimolecular fluorescence complementation. Nat. Methods 3, 597–600 (2006).
Granseth, B., Odermatt, B., Royle, S.J. & Lagnado, L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51, 773–786 (2006).
Zhu, Y., Xu, J. & Heinemann, S.F. Two pathways of synaptic vesicle retrieval revealed by single-vesicle imaging. Neuron 61, 397–411 (2009).
Li, H. et al. Concurrent imaging of synaptic vesicle recycling and calcium dynamics. Front. Mol. Neurosci. 4, 34 (2011).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 (2009).
Boron, W.F. & De Weer, P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J. Gen. Physiol. 67, 91–112 (1976).
Reuter, H. Measurements of exocytosis from single presynaptic nerve terminals reveal heterogeneous inhibition by Ca2+-channel blockers. Neuron 14, 773–779 (1995).
Reid, C.A., Clements, J.D. & Bekkers, J.M. Nonuniform distribution of Ca2+ channel subtypes on presynaptic terminals of excitatory synapses in hippocampal cultures. J. Neurosci. 17, 2738–2745 (1997).
Murthy, V.N. & Stevens, C.F. Reversal of synaptic vesicle docking at central synapses. Nat. Neurosci. 2, 503–507 (1999).
Pierce, J.P. & Lewin, G.R. An ultrastructural size principle. Neuroscience 58, 441–446 (1994).
Harris, K.M. & Stevens, J.K. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 9, 2982–2997 (1989).
Branco, T., Staras, K., Darcy, K.J. & Goda, Y. Local dendritic activity sets release probability at hippocampal synapses. Neuron 59, 475–485 (2008).
Voglmaier, S.M. et al. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron 51, 71–84 (2006).
Ramirez, D.M., Khvotchev, M., Trauterman, B. & Kavalali, E.T. Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission. Neuron 73, 121–134 (2012).
Johnson, D.E. et al. Red fluorescent protein pH biosensor to detect concentrative nucleoside transport. J. Biol. Chem. 284, 20499–20511 (2009).
Tantama, M., Hung, Y.P. & Yellen, G. Imaging intracellular pH in live cells with a genetically encoded red fluorescent protein sensor. J. Am. Chem. Soc. 133, 10034–10037 (2011).
Micheva, K.D., Buchanan, J., Holz, R.W. & Smith, S.J. Retrograde regulation of synaptic vesicle endocytosis and recycling. Nat. Neurosci. 6, 925–932 (2003).
Auld, D.S. & Robitaille, R. Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron 40, 389–400 (2003).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Rickgauer, J.P. & Tank, D.W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl. Acad. Sci. USA 106, 15025–15030 (2009).
Shaner, N.C., Steinbach, P.A. & Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2, 905–909 (2005).
Zhang, Q., Li, Y. & Tsien, R.W. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science 323, 1448–1453 (2009).
Kao, J.P., Harootunian, A.T. & Tsien, R.Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J. Biol. Chem. 264, 8179–8184 (1989).
Maravall, M., Mainen, Z.F., Sabatini, B.L. & Svoboda, K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys. J. 78, 2655–2667 (2000).
Acknowledgements
We thank M. Lin for help with quantum yield measurements. We thank J. Emery for technical assistance and L. Li for help with transfections. We thank L. Luo and Tsien laboratory members, especially M. Tadross, S. Owen, H. Park and R. Groth, for discussions throughout the execution of this project. This work was supported by grants from the US National Institute of Neurological Disorders and Stroke (NS074785 and NS24067), the US National Institute of Mental Health (MH064070), the Mathers Foundation and the Burnett family fund (to R.W.T.).
Author information
Authors and Affiliations
Contributions
Y.L. conceived and performed the experiments. R.W.T. supervised the project. Y.L. and R.W.T. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 2519 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Tsien, R. pHTomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity. Nat Neurosci 15, 1047–1053 (2012). https://doi.org/10.1038/nn.3126
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3126
This article is cited by
-
An active tethering mechanism controls the fate of vesicles
Nature Communications (2021)
-
pHmScarlet is a pH-sensitive red fluorescent protein to monitor exocytosis docking and fusion steps
Nature Communications (2021)
-
Molecular tools for imaging and recording neuronal activity
Nature Chemical Biology (2019)
-
Brain activity regulates loose coupling between mitochondrial and cytosolic Ca2+ transients
Nature Communications (2019)
-
NTnC-like genetically encoded calcium indicator with a positive and enhanced response and fast kinetics
Scientific Reports (2018)