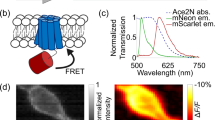
Abstract
Genetically encoded voltage indicators (GEVIs) enable optical recording of electrical signals in the brain, providing subthreshold sensitivity and temporal resolution not possible with calcium indicators. However, one- and two-photon voltage imaging over prolonged periods with the same GEVI has not yet been demonstrated. Here, we report engineering of ASAP family GEVIs to enhance photostability by inversion of the fluorescence–voltage relationship. Two of the resulting GEVIs, ASAP4b and ASAP4e, respond to 100-mV depolarizations with ≥180% fluorescence increases, compared with the 50% fluorescence decrease of the parental ASAP3. With standard microscopy equipment, ASAP4e enables single-trial detection of spikes in mice over the course of minutes. Unlike GEVIs previously used for one-photon voltage recordings, ASAP4b and ASAP4e also perform well under two-photon illumination. By imaging voltage and calcium simultaneously, we show that ASAP4b and ASAP4e can identify place cells and detect voltage spikes with better temporal resolution than commonly used calcium indicators. Thus, ASAP4b and ASAP4e extend the capabilities of voltage imaging to standard one- and two-photon microscopes while improving the duration of voltage recordings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Sequences of ASAP4b and ASAP4e have been deposited to GenBank with accession numbers OQ822155 and OQ822156, respectively. Plasmids for expression or AAV packaging of ASAP4b-Kv and ASAP4e-Kv, along with their full sequences, are available at addgene.org with accession numbers 201030 and 201031 (constitutive expression in neurons from the synapsin promoter of ASAP4b-Kv and ASAP4e-Kv, respectively), and 201032 and 201033 (cre-dependent expression from the EF1α promoter of ASAP4b-Kv and ASAP4e-Kv, respectively). Packaged AAVs expressing ASAP4b-Kv and ASAP4e-Kv are also available from the Stanford Gene and Viral Vector Core. Raw image datasets are available at zenodo.org with record number 8059957.
Code availability
The analysis code used in this study is available at github.com/swenceslaoe/Final_ASAP4_paper_detection_code under the terms of the open source Apache License 2.0.
References
Antic, S. D., Empson, R. M. & Knöpfel, T. Voltage imaging to understand connections and functions of neuronal circuits. J. Neurophysiol. 116, 135–152 (2016).
Zhu, M. H., Jang, J., Milosevic, M. M. & Antic, S. D. Population imaging discrepancies between a genetically-encoded calcium indicator (GECI) versus a genetically-encoded voltage indicator (GEVI). Sci. Rep. 11, 5295 (2021).
Helassa, N., Podor, B., Fine, A. & Török, K. Design and mechanistic insight into ultrafast calcium indicators for monitoring intracellular calcium dynamics. Sci. Rep. 6, 38276 (2016).
Yang, H. H. & St-Pierre, F. Genetically encoded voltage indicators: opportunities and challenges. J. Neurosci. 36, 9977–9989 (2016).
Zhang, Y. et al. Fast and sensitive GCaMP calcium indicators for imaging neural populations. Nature 615, 884–891 (2023).
Brinks, D., Klein, A. J. & Cohen, A. E. Two-photon lifetime imaging of voltage indicating proteins as a probe of absolute membrane voltage. Biophys. J. 109, 914–921 (2015).
Villette, V. et al. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice. Cell 179, 1590–1608.e23 (2019).
Wu, J. et al. Kilohertz two-photon fluorescence microscopy imaging of neural activity in vivo. Nat. Methods 17, 287–290 (2020).
Webb, B. & Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinformatics 54, 5.6.1–5.6.37 (2016).
Abdelfattah, A. S. et al. A general approach to engineer positive-going eFRET voltage indicators. Nat. Commun. 11, 3444 (2020).
Barnett, L., Platisa, J., Popovic, M., Pieribone, V. A. & Hughes, T. A fluorescent, genetically-encoded voltage probe capable of resolving action potentials. PLoS ONE 7, e43454 (2012).
Milosevic, M. M., Jang, J., McKimm, E. J., Zhu, M. H. & Antic, S. D. In vitro testing of voltage indicators: Archon1, ArcLightD, ASAP1, ASAP2s, ASAP3b, Bongwoori-Pos6, BeRST1, FlicR1, and Chi-VSFP-Butterfly. eNeuro 7, ENEURO.0060-20.2020 (2020).
Platisa, J., Vasan, G., Yang, A. & Pieribone, V. A. Directed evolution of key residues in fluorescent protein inverses the polarity of voltage sensitivity in the genetically encoded indicator ArcLight. ACS Chem. Neurosci. 8, 513–523 (2017).
Han, Z. et al. Mechanistic studies of the genetically encoded fluorescent protein voltage probe ArcLight. PLoS ONE 9, e113873 (2014).
Akerboom, J. et al. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J. Biol. Chem. 284, 6455–6464 (2009).
Lam, A. J. et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 9, 1005–1012 (2012).
Piatkevich, K. D. et al. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat. Chem. Biol. 14, 352–360 (2018).
Ning, L. et al. A bright, nontoxic, and non-aggregating red fluorescent protein for long-term labeling of fine structures in neurons. Front. Cell Dev. Biol. 10, 893468 (2022).
Daigle, T. L. et al. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell 174, 465–480.e22 (2018).
Lim, S. T., Antonucci, D. E., Scannevin, R. H. & Trimmer, J. S. A novel targeting signal for proximal clustering of the Kv2.1 K+ channel in hippocampal neurons. Neuron 25, 385–397 (2000).
Fan, J. L. et al. High-speed volumetric two-photon fluorescence imaging of neurovascular dynamics. Nat. Commun. 11, 6020 (2020).
Wilt, B. A., Fitzgerald, J. E. & Schnitzer, M. J. Photon shot noise limits on optical detection of neuronal spikes and estimation of spike timing. Biophys. J. 104, 51–62 (2013).
Huh, C. Y. et al. Excitatory inputs determine phase-locking strength and spike-timing of CA1 stratum oriens/alveus parvalbumin and somatostatin interneurons during intrinsically generated hippocampal theta rhythm. J. Neurosci. 36, 6605–6622 (2016).
Dana, H. et al. Sensitive red protein calcium indicators for imaging neural activity. eLife 5, e12727 (2016).
Dana, H. et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657 (2019).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Huang, L. et al. Relationship between simultaneously recorded spiking activity and fluorescence signal in GCaMP6 transgenic mice. eLife 10, e51675 (2021).
Koester, H. J. & Sakmann, B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J. Physiol. 529, 625–646 (2000).
White, M. D., Milne, R. V. & Nolan, M. F. A molecular toolbox for rapid generation of viral vectors to up- or down-regulate neuronal gene expression in vivo. Front. Mol. Neurosci. 4, 8 (2011).
Zolotukhin, S. et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6, 973–985 (1999).
Zhang, D. Y., Lau, C. P. & Li, G. R. Human Kir2.1 channel carries a transient outward potassium current with inward rectification. Pflug. Arch. 457, 1275–1285 (2009).
Fan, L. Z. et al. All-optical synaptic electrophysiology probes mechanism of ketamine-induced disinhibition. Nat. Methods 15, 823–831 (2018).
Pfeiffer, B. D. et al. Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755 (2010).
Rister, J. et al. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron 56, 155–170 (2007).
Edelstein, A. D., Tsuchida, M. A., Amodaj, N., Pinkard, H., Vale, R. & Stuurman, N. Advanced methods of microscope control using æManager software. J. Biol. Methods 1, e10 (2014).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Jia, H., Rochefort, N. L., Chen, X. & Konnerth, A. Dendritic organization of sensory input to cortical neurons in vivo. Nature 464, 1307–1312 (2010).
Yang, H. H. et al. Subcellular imaging of voltage and calcium signals reveals neural processing in vivo. Cell 166, 245–257 (2016).
Liu, Z. et al. Sustained deep-tissue voltage recording using a fast indicator evolved for two-photon microscopy. Cell 185, 3408–3425.e29 (2022).
Makinson, C. D. et al. Regulation of thalamic and cortical network synchrony by Scn8a. Neuron 93, 1165–1179.e6 (2017).
Ding, J., Peterson, J. D. & Surmeier, D. J. Corticostriatal and thalamostriatal synapses have distinctive properties. J. Neurosci. 28, 6483–6492 (2008).
Carter, A. G. & Sabatini, B. L. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron 44, 483–493 (2004).
Sun, W., Tan, Z., Mensh, B. D. & Ji, N. Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nat. Neurosci. 19, 308–315 (2016).
Xu, F., Shi, D. Q., Lau, P. M., Lin, M. Z. & Bi, G. Q. Excitation wavelength optimization improves photostability of ASAP-family GEVIs. Mol. Brain 11, 32 (2018).
Podgorski, K. & Ranganathan, G. Brain heating induced by near-infrared lasers during multiphoton microscopy. J. Neurophysiol. 116, 1012–1023 (2016).
Taxidis, J. et al. Differential emergence and stability of sensory and temporal representations in context-specific hippocampal sequences. Neuron 108, 984–998.e9 (2020).
Hwang, F. J. et al. Motor learning selectively strengthens cortical and striatal synapses of motor engram neurons. Neuron 110, 2790–2801.e5 (2022).
Chien, M. P. et al. Photoactivated voltage imaging in tissue with an archaerhodopsin-derived reporter. Sci. Adv. 7, eabe3216 (2021).
Plitt, M. H. & Giocomo, L. M. Experience-dependent contextual codes in the hippocampus. Nat. Neurosci. 24, 705–714 (2021).
Skaggs, W., Mcnaughton, B. & Gothard, K. An information-theoretic approach to deciphering the hippocampal code. Adv. Neural Inf. Process. Syst. 5, 1030–1030 (1992).
Acknowledgements
We thank L. Liu (Lin Lab, Stanford University) for technical assistance, D. Ringach (Neurolabware) for help with firmware changes to the two-photon microscope used for in vivo hippocampal imaging in the Giocomo Lab, H. Yang and M. Xie (Cladinin Lab, Stanford) for the stimulus generation and data analysis code used in the fly experiments, C. Yang and M. Melin (Golshani Lab, UCLA) for mouse surgical preparations for one-photon hippocampal imaging, and N. Wall and the Stanford Gene and Viral Vector Core for AAV packaging. Funding was provided by a Human Frontier Science Program Long-term Fellowship grant no. LT000916/2018-L (C.-M.S.); the NDSEG Fellowship Program (M.M.P.); a Parkinson’s Foundation Postdoctoral Fellowship grant no. PF-FBS-2027 (R.H.R.); an American Epilepsy Society Wishes for Elliott Predoctoral Research Fellowship (M.C.); a Stanford Bio-X Graduate Fellowship (Y.A.H.); a Stanford MCHRI Postdoctoral Support award (D.J.); a Stanford MCHRI Uytengsu-Hamilton 22q11 Neuropsychiatry Research Award (M.Z.L.); Stanford University Wu Tsai Neurosciences Institute Seed Grant no. 133808 (R.O.D. and M.Z.L.); Post-9/11 GI Bill and NIH grant no. 5T32MH020016 (S.W.E.); CAS grant no. XDB32030200 and Key-Area R&D Program of Guangdong Province grant no. 2018B030331001 (G.-Q.B.); Office of Naval Research grant no. N00141812690, Simons Foundation grant no. SCGB 542987SPI and the James S. McDonnell Foundation (M.H.P. and L.M.G.); the Vallee Foundation (L.M.G.); NIH grants no. 1R01MH124047, no. 1R01MH124867, no. 1U19NS104590 and no. 1U01NS115530 (A.L. and A.N.); and NIH grants no. UF1NS107696 (N.J. and J.L.F.), no. R00NS104215 (C.D.M.), no. R01NS116589 (P.G.), no. R01EY022638 (M.M.P., S.S. and T.R.C.), no. 5U01NS103464 (S.L. and M.Z.L.), no. 1R01MH114227 (M.Z.L. and J.B.D.), no. 1RF1MH11410501 (M.Z.L., J.B.D. and T.R.C.) and no. 1R01NS123681 (M.Z.L., J.B.D., T.R.C., L.M.G., N.J. and P.G.).
Author information
Authors and Affiliations
Contributions
Library design, construction and screening were done by L.P., M.C., D.-Q.S. and S.W.E. with supervision by M.Z.L. Patch-clamping of cultured cells was done by M.C., D.-Q.S., D.J. and G.Z. with supervision by M.Z.L. and G.-Q.B. Molecular modeling was done by S.C.V.K. and C.-M.S. with supervision by R.O.D. The in vitro photobleaching was done by S.L., Y.A.H. and S.W.E. with supervision by M.Z.L. The membrane localization experiment and analysis were done by D.J. and Y.A.H. with supervision by M.Z.L. Hippocampal voltage waveforms were recorded in vivo by A.N. with supervision by A.L. Fly experiments were performed by M.M.P. and S.S. with supervision by T.R.C. Some AAV packaging was performed by Y.L. with supervision by S.W. Striatal acute slice brightness measurements were done by S.W.E. with supervision by M.Z.L. and J.B.D. Hippocampal acute slice electrophysiology and imaging were done by F.-J.H. and S.W.E. with supervision by J.B.D. and by A.L.R. with supervision by C.D.M. The in vivo one-photon imaging of hippocampal neurons was performed by J.T., B.M. and C.C.D. with supervision by P.G., and of layer-1 neurons in motor cortex by S.L., F.-J.H. and R.H.R. with supervision by M.Z.L. and J.B.D. The one-photon spike detection method was developed by S.W.E. The in vivo two-photon cortical neuron imaging and analysis were done by J.L.F. with supervision by N.J. The in vivo two-photon hippocampal place cell imaging was done by M.H.P. with supervision by L.M.G. Additional data analyses and manuscript preparation were done by S.W.E., S.L., Y.A.H. and M.Z.L. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.Z.L. is an inventor on a patent for the earlier ASAP1 voltage indicator. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Michael Häusser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Nina Vogt, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Reversing the fluorescence response.
(A) Peak responses following electroporation of HEK293-Kir2.1 cells transfected with PCR-assembled genes encoding all 400 combinations of 20 amino acids each at positions 150 and 151 in ASAP2f L146G S147T 414Q. Note some responses were negative (dimming) and others were positive (brightening). Mutant 150D 151 G and mutant 150 T 151 T showed the largest positive responses. (B) Left, relative fluorescence changes from –70 mV of HEK293A cells transfected with plasmids expressing mutant 150D 151 G or mutant 150 T 151 T at various command voltages during patch-clamp electrophysiology. Mean responses normalized to –70 mV are plotted. Error bars represent standard error of the mean (SEM) of n = 8 cells in each group. Right, activation kinetics in response to 2-ms and 1-s steps from –70 mV to +30 mV of mutant 150D 151 G or mutant 150 T 151 T. Gray band represents SEM of n = 8 cells in each group. Mutant 150D 151 G was designated ASAP4.0.
Extended Data Fig. 2 ASAP4.0 to ASAP4.2.
(A) Steady-state responses from command voltage patch clamping in HEK 293 A cells for ASAP4.0 to ASAP4.2. Responses are normalized to –70 mV. The test pulses before each step are 2-ms square steps from –70 to +30 mV (B) Left, ∆F/F0 over voltage steps for the parent construct ASAP2f L146G S147T R414Q, as well as ASAP3 and the first three ASAP4 variants. Error bars are SEM. Right, brightness vs. voltage plotted as a fraction of the maximum brightness for each indicator. Data are displayed as mean ± SEM. The gray box highlights the physiological range from –70 to +30 mV. (C) Screening all combinations of F148X N149X in ASAP4.1 (ASAP4.0 T206H) yielded F148P N149V, creating ASAP4.2.
Extended Data Fig. 3 Improvements to ASAP4.2.
(A) Mean fluorescent responses of ASAP4.2 F413X variants to a series of voltage steps from –120 to +50 mV, from a holding potential of –70 mV in voltage-clamped HEK293A cells. Error bars represent SEM. Only proline was excluded due to expression issues. (B) F/Fmax a 23 °C for ASAP4.2 and the three best derivatives. Error bars represent SEM centered on measured mean values; curved lines represent Boltzmann distributions fit to the measured mean values. (C) F/Fmax at 23 and 37 °C for ASAP3, ASAP4b, and ASAP4e. Error bars represent SEM. (D) F/Fmax in HEK cells under 2-photon illumination at 940 nm for ASAP3, ASAP4b, and ASAP4e imaged at 23 °C. n = 73 for ncpASAP4b, 81 for ASAP3, 126 for ASAP4b, and 76 for ASAP4e. Bars represent mean ± SEM. All GEVIs were significantly different from each other (p < 0.0001), except for ASAP4b vs. ASAP4e (p = 0.8108) by Dunnett’s multiple comparison test. (E) One-photon excitation and emission spectra measured for ASAP3, ASAP4b, and ASAP4e in HEK293-Kir2.1 cells at 23 °C. (F) Two-photon excitation spectra measured with 2-nm spectral intervals from HEK293-Kir2.1 cells. Peaks were at 938 nm. n = 52 for ncpASAP4b, 57 for ASAP3, 57 for ASAP4b, and 44 for ASAP4e.
Extended Data Fig. 4 Photobleaching of ASAP indicators under one-photon and two-photon excitation.
(A) One-photon photobleaching curves over 5 min of continuous illumination in cultured HEK293-Kir2.1 cells, using blue light with a peak wavelength at 453 nm, tested with varying intensities. Shaded areas represent SEM. (B) Resonant-scanning two-photon photobleaching in HEK293-Kir2.1 cells at 518 and 888 mW/mm2). Shaded areas represent SEM. (C) Galvanometric-scanning two-photon photobleaching in HEK293-Kir2.1 cells at 960 mW/mm2. After 5 min, cells were incubated the dark and imaged at single time points 2.7, 3.3, and 5.0 min later. Shaded areas represent SEM.
Extended Data Fig. 5 Responses of ASAP family GEVIs to AP waveforms in HEK293A cells.
(A) Responses of indicators in HEK293a cells to an AP waveform (far left) under voltage clamp that has been modified to have either a 2 ms FWHM (top), or a 4 ms FWHM (bottom), and ranges from -70mV to +30 mV. Each cell had the waveform applied 5 times, with n = 6 cells for ASAP3, n = 7 cells for ASAP4b, n = 5 for ASAP4e, and n = 7 cells for Upward Ace2mNeon. Shading is SEM. (B) Response to commanded spike trains in HEK293A cells at room temp. Vm was –70 mV at the start of the displayed segment and peak AP amplitude was 100 mV. Responses were collected from 6 (ASAP4.2), 7 (ASAP4b), 5(ASAP4e), 6 (ASAP3) and 7 (Upward Ace2mNeon) HEK293A cells, each supplying a single unfiltered and unsmoothed 1000-Hz trace. Colored lines represent averages, gray shading is SEM. (C) Responses of indicators in HEK293A cells an AP burst waveforms of 1–4 spikes with spike widths of 1.0 ms at FWHM. These waveforms were previously recorded from CA1 mouse hippocampal pyramidal neurons in vivo. Vm was –52 mV at the start of the displayed voltage segment. Each colored line is a single unfiltered trace with no trial averaging or filtering applied.
Extended Data Fig. 6 Imaging ASAP4b and ASAP2f in awake flies during visual stimulation.
(A) From top to bottom: (i) Experimental setup for two-photon imaging of visually evoked responses in the Drosophila brain using a flickering visual stimulus on a gray background. (ii) Drawing depicting L2 non-spiking interneurons. L2 interneurons are directly postsynaptic to photoreceptors R1–R6, and depolarize to dark flashes and hyperpolarize to light flashes. Multiple L2 interneurons send axons in parallel through the lamina into the medulla, tiling visual space. (iii) Sample two-photon image of ASAP4b expression in the terminal arbors of four L2 axons. (B) Stimulus-evoked GEVI responses in L2 terminal arbors (n = 43 neurons from five ASAP2f flies, or 45 neurons from four ASAP4b flies). The ASAP2f response is shown inverted to facilitate comparison. Flies were stimulated with a repeated sequence of 20-ms light and dark flashes starting from a mean gray background, and then, for each arbor, stimuli-aligned responses were extracted and averaged. The solid line in the graph represents the mean waveform across all cells sampled, and the shaded area represents SEM. (C) Left, mean responses of ASAP4b or ASAP2f to depolarizing or hyperpolarizing stimuli. Error bars represent SEM. *** p < 0.001 for depolarization (dark) and for hyperpolarization (light), using a two-sided t-test. Right, ASAP4b produces responses with faster kinetics than ASAP2f. Plots represent mean ± SEM. * p = 0.0406 for depolarization (dark) and 0.0268 for hyperpolarization (light), by two-sided t-test.
Extended Data Fig. 7 Expression of pan-membrane and somatically enriched ASAP4b and ASAP4e in neurons.
(A) Example images from 15-DIV cortical neurons co-expressing ASAPs in green channel and farnesylated Crimson in red channel. (B) Quantification of membrane expression for unfused ASAP3 (n = 19), ASAP4b (n = 17), and ASAP4e (n = 16). Statistical significance was determined by one-way ANOVA followed by post-hoc Tukey test. Error bars represent SEM. (C) ASAP4b and ASAP4e expression in cultured hippocampal neurons from lipofection (14–15 DIV). This experiment with three independent cultures and transfections. (D) Examples of ASAP3-Kv, ASAP4b-Kv, and ASAP4e-Kv in acute striatal slices under one-photon illumination. Scale bar is 20 μM. (E) Brightness of striatal medium spiny neurons expressing somatically localized ASAPs under one-photon illumination in acute slice. n = 36 cells for ASAP3-Kv, 18 for ASAP4b-Kv, and 18 for ASAP4e-Kv, with each mouse providing 6 cells chosen randomly. Bars indicate mean ± SEM. Differences were insignificant by Kruskal Wallis test. (F) Brightness of SST+ hippocampal interneurons expressing somatically localized ASAPs under one-photon illumination in vivo. n = 28 cells for ASAP3-Kv, 13 for ASAP4b-Kv, and 17 for ASAP4e-Kv from 2–4 mice for each GEVI. Error bars are SEM. Differences were insignificant by Kruskal Wallis test. (G) Brightness of patch-clamped hippocampal CA1 pyramidal neurons expressing somatically localized ASAPs under 940-nm two-photon illumination. Bars indicate mean ± SEM. n = 6 cells for ASAP3-Kv and 7 for ASAP4b-Kv, with one cell per mouse. Differences in brightness were insignificant by two-sided Mann Whitney U test. (H) Long-term ASAP4e-Kv expression in the mouse brain. AAV9-EF1α-DiO-ASAP-WPRE and AAV9-synapsin-Cre were co-injected at at 1000:1 ratio in motor cortex, then 7 months later the brain was fixed and sectioned. Direct ASAP4e fluorescence was integrated through 18 optical sections spaced 4 μm apart.
Extended Data Fig. 8 Detection of 50-Hz AP trains in acute hippocampal slice.
(A) One-photon imaging of patch-clamped CA1 neurons in acute hippocampal slices expressing either ASAP3-Kv (n = 4 cells) or ASAP4b-Kv (n = 4 cells). Spike trains were evoked with current pulses, and voltage waveforms and GEVI (1000 frames per second) were recorded. Mean unfiltered responses of 12 sweeps in total are displayed with gray areas representing SEM. (B) Empirical SNR (ΔF divided by SD of F0) for single-AP one-photon responses of ASAP3-Kv and ASAP4b-Kv during spike trains. Mean ± SD is plotted. A Kruskal-Wallis test followed by Dunn’s multiple comparison test was used to compare ASAP4b-Kv to ASAP3-Kv for each spiking frequency, revealing significantly higher SNR of ASAP3-Kv at 50 Hz (multiplicity-adjusted p < 0.0001 from 522 spikes for ASAP3-Kv and 351 spikes for ASAP4b-Kv). (C) Two-photon imaging of pyramidal neurons in acute hippocampal slices expressing either ASAP3-Kv (n = 6 cells, 21 sweeps total) or ASAP4b-Kv (n = 5 cells, 15 sweeps total). Mean unfiltered responses are displayed with gray areas representing SEM. (D) Empirical SNR (ΔF divided by SD of F0) for single-AP one-photon responses of ASAP3-Kv and ASAP4b-Kv during spike trains. Mean ± SD is plotted. A Kruskal-Wallis test followed by Dunn’s multiple comparison test was used to compare ASAP4b-Kv to ASAP3-Kv for each spiking frequency, revealing significantly higher SNR for ASAP4b-Kv at all frequencies (multiplicity-adjusted p < 0.0001, from 475 spikes for ASAP3-Kv and 375 spikes for ASAP4b-Kv).
Extended Data Fig. 9 ASAP4b-Kv responses to spikes evoked by current steps in hippocampal CA1 pyramidal neurons under two-photon imaging.
(A) Above: raw, uncorrected fluorescence traces, showing example single-trial fluorescence responses to the two indicators in our 2 P system for ASAP4b-kv (blue) and ASAP3-kv (green). Below, the corresponding electrophysiological traces for the raw fluorescence traces shown above. (B) Empirical SNR calculated as the ratio of ΔF/F0 to the SD of F0 for current step-evoked spikes. Error bars are SEM. ASAP4b-Kv had significantly higher SNR than ASAP3-Kv (p < 0.0001 by two-sided Mann-Whitney U test). Error bars are SEM. n = 611 spikes for ASAP3-Kv and 284 for ASAP4b-Kv. (C) Average ASAP3-Kv and ASAP4e-Kv fluorescence responses to current step-evoked spikes of at least 20 mV, overlaid over the average voltage response. Time from crossing 20 mV crossing to fluorescence peak was 1.3 ± 1.4 ms (SD) for ASAP3-Kv and 3.6 + 1.3 ms for ASAP4b-Kv. Sampling rate was 1 kHz for fluorescence and 10 kHz for electrophysiology. Error bars are SEM. n = 611 spikes for ASAP3-Kv and 284 for ASAP4e-Kv. (D) Histogram of the interspike-intervals (ISIs) for each indicator with 10-ms bins. Circles represent actual histogram values.
Extended Data Fig. 10 In vivo voltage imaging under one-photon wide-field illumination in hippocampus and primary motor cortex.
(A) Photostability during continuous one-photon illumination of ASAP3-Kv, ASAP4b-Kv, and ASAP4e-Kv in hippocampal SST+ interneurons in the mouse brain 4–7 weeks post-infection. Powers used were 100–250 mW/mm2, and time coordinates were adjusted to simulate 100 mW/mm2 assuming linearity between power and photobleaching rate. (B) A hippocampal CaMK2α + neuron expressing ASAP4b-Kv 4 weeks post-infection, imaged under one-photon illumination (150 mW/mm2) and its fluorescence trace for 30 seconds. The signals were acquired at 1000-fps and time-binned to 500fps. The trace was normalized to the mean signal of the first 100 frames and not corrected for photobleaching. The right-most zoomed-in trace shows spontaneous spikes.
Supplementary information
Supplementary Information
Supplementary Note and Table 1.
Supplementary Video 1
In vivo imaging of ASAP4e-kv and jRGECO1a in mouse V1. Neurons coexpressing ASAP4e-Kv and jRECO1a were recorded by two-photon scanning at 99 frames per second with a single 1,000-nm excitation wavelength, with green (ASAP4e-Kv) and red (jRECO1a) emissions directed to separate detectors by a beam-splitter. Above, raw image data. The white arrow points to the quantified cell. Below, plots of ∆F/F0 in green and red channels. Traces are corrected for photobleaching for display purposes but unfiltered. The same traces uncorrected for photobleaching can be seen in Fig. 5. ASAP4e-Kv fluorescence changes of ∆F/F0 >3 s.d. from the baseline are marked visually by ticks above the trace and aurally by a click sound.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Evans, S.W., Shi, DQ., Chavarha, M. et al. A positively tuned voltage indicator for extended electrical recordings in the brain. Nat Methods 20, 1104–1113 (2023). https://doi.org/10.1038/s41592-023-01913-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-023-01913-z
This article is cited by
-
More than double the fun with two-photon excitation microscopy
Communications Biology (2024)
-
Kirigami electronics for long-term electrophysiological recording of human neural organoids and assembloids
Nature Biotechnology (2024)
-
Lighting up action potentials with fast and bright voltage sensors
Nature Methods (2023)