Abstract
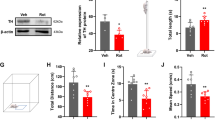
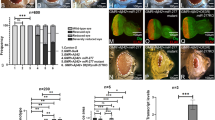
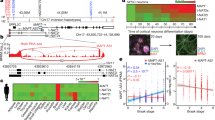
Spinocerebellar ataxia type 1 is caused by expansion of a translated CAG repeat in ataxin1 (ATXN1). The level of the polyglutamine-expanded protein is one of the factors that contributes to disease severity. Here we found that miR-19, miR-101 and miR-130 co-regulate ataxin1 levels and that their inhibition enhanced the cytotoxicity of polyglutamine-expanded ATXN1 in human cells. We provide a new candidate mechanism for modulating the pathogenesis of neurodegenerative diseases sensitive to protein dosage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Orr, H.T. & Zoghbi, H.Y. Annu. Rev. Neurosci. 30, 575–621 (2007).
Orr, H.T. et al. Nat. Genet. 4, 221–226 (1993).
Banfi, S. et al. Nat. Genet. 7, 513–520 (1994).
Burright, E.N. et al. Cell 82, 937–948 (1995).
Cemal, C.K. et al. Hum. Mol. Genet. 11, 1075–1094 (2002).
Xia, H. et al. Nat. Med. 10, 816–820 (2004).
Huynh, D.P., Figueroa, K., Hoang, N. & Pulst, S.M. Nat. Genet. 26, 44–50 (2000).
Bartel, D.P. Cell 116, 281–297 (2004).
Lewis, B.P., Shih, I.H., Jones-Rhoades, M.W., Bartel, D.P. & Burge, C.B. Cell 115, 787–798 (2003).
Krek, A. et al. Nat. Genet. 37, 495–500 (2005).
Schaefer, A. et al. J. Exp. Med. 204, 1553–1558 (2007).
Zoghbi, H.Y. & Orr, H.T. Semin. Cell Biol. 6, 29–35 (1995).
Clark, H.B. et al. J. Neurosci. 17, 7385–7395 (1997).
Rich, T. & Varadaraj, A. PLoS ONE 2, e1014 (2007).
Lim, J. et al. Nature 452, 713–718 (2008).
Acknowledgements
We thank N. Ao, Y. Liu and A. Liang of the Baylor College of Medicine In Situ Hybridization core for technical assistance and V.N. Kim and members of the Zoghbi laboratory for helpful discussions and comments on the manuscript. This research was supported by US National Institutes of Health grants NS27699 and HD24064 to H.Y.Z. and NS22920 to H.T.O. H.Y.Z. is an investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
Y.L. and H.Y.Z. designed the experiments. Y.L. carried out the majority of the experiments, with the exception of those comparing SCA1[82Q]Tg/+ and SCA1[82Q]Tg/Tg mice in Supplementary Figure 1 (J.R.G. and R.C.S.) and the RNA in situ hybridization experiments in Figure 2 (C.T.). Data analyses and interpretation were conducted by Y.L., H.T.O. and H.Y.Z. Y.L. and H.Y.Z. wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Methods (PDF 7176 kb)
Rights and permissions
About this article
Cite this article
Lee, Y., Samaco, R., Gatchel, J. et al. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat Neurosci 11, 1137–1139 (2008). https://doi.org/10.1038/nn.2183
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2183
This article is cited by
-
The role of microRNAs in neurodegenerative diseases: a review
Cell Biology and Toxicology (2023)
-
Modifier pathways in polyglutamine (PolyQ) diseases: from genetic screens to drug targets
Cellular and Molecular Life Sciences (2022)
-
SRSF10 inhibits biogenesis of circ-ATXN1 to regulate glioma angiogenesis via miR-526b-3p/MMP2 pathway
Journal of Experimental & Clinical Cancer Research (2020)
-
Genetics, Mechanisms, and Therapeutic Progress in Polyglutamine Spinocerebellar Ataxias
Neurotherapeutics (2019)
-
Moving Towards Therapy in SCA1: Insights from Molecular Mechanisms, Identification of Novel Targets, and Planning for Human Trials
Neurotherapeutics (2019)