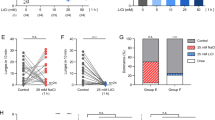
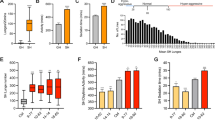
Abstract
Aggression is an innate behavior that is important for animal survival and evolution. We examined the molecular and cellular mechanisms underlying aggression in Drosophila. Reduction of the neurotransmitter octopamine, the insect equivalent of norepinephrine, decreased aggression in both males and females. Mutants lacking octopamine did not initiate fighting and did not fight other flies, although they still provoked other flies to fight themselves. Mutant males lost to the wild-type males in fighting and in competing for copulation with females. Enhanced octopaminergic signaling increased aggression in socially grouped flies, but not in socially isolated flies. We carried out genetic rescue experiments that revealed the functional importance of neuronal octopamine and identified a small subset of octopaminergic neurons in the suboesophageal ganglion as being important for aggression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Darwin, C. The Descent of Man and Selection in Relation to Sex (John Murray, London, England, 1871).
Lorenz, K.Z. On Aggression (Harcourt, Brace and World, New York, 1963).
Scott, J.P. Genetic differences in the social behavior of inbred strains of mice. J. Hered. 33, 11–15 (1942).
Brunner, H.G., Nelen, M., Breakefield, X.O., Ropers, H.H. & van Oost, B.A. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262, 578–580 (1993).
Sturtevant, A.H. Experiments on sex recognition and the problem of sexual selection in Drosophila. Anim. Behav. 5, 351–366 (1915).
Jacobs, M.E. Influence of light on mating of Drosophila melanogaster. Ecology 41, 182–188 (1960).
Dow, M.A. & von Schilcher, F. Aggression and mating success in Drosophila melanogaster. Nature 254, 511–512 (1975).
Jacobs, M.E. Influence of β-alanine on mating and territorialism in Drosophila melanogaster. Behav. Genet. 8, 487–502 (1978).
Hoffmann, A.A. A laboratory study of male territoriality in the sibling species Drosophila melanogaster and D. simulans. Anim. Behav. 35, 807–818 (1987).
Hoffmann, A.A. Territorial encounters between Drosophila males of different sizes. Anim. Behav. 35, 1899–1901 (1987).
Hoffmann, A.A. Geographic variation in the territorial success of Drosophila melanogaster males. Behav. Genet. 19, 241–255 (1989).
Hoffmann, A.A. The influence of age and experience with conspecifics on territorial behaviour in Drosophila melanogaster. J. Insect Behav. 3, 1–12 (1990).
Hoffmann, A.A. & Cacoyianni, Z. Selection for territoriality in Drosophila melanogaster: correlated responses in mating success and other fitness components. Anim. Behav. 38, 23–34 (1989).
Hoffmann, A.A. & Cacoyianni, Z. Territoriality in Drosophila melanogaster as a conditional strategy. Anim. Behav. 40, 526–537 (1990).
Ueda, A. & Kidokoro, Y. Aggressive behaviours of female Drosophila melanogaster are influenced by their social experience and food resources. Physiol. Entomol. 27, 21–28 (2002).
Nilsen, S.P., Chan, Y.B., Huber, R. & Kravitz, E.A. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 101, 12342–12347 (2004).
Vrontou, E., Nilsen, S.P., Demir, E., Kravitz, E.A. & Dickson, B.J. fruitless regulates aggression and dominance in Drosophila. Nat. Neurosci. 9, 1469–1471 (2006).
Dierick, H.A. & Greenspan, R.J. Molecular analysis of flies selected for aggressive behavior. Nat. Genet. 38, 1023–1031 (2006).
Edwards, A.C., Rollmann, S.M., Morgan, T.J. & Mackay, T.F. Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2, e154 (2006).
Dierick, H.A. & Greenspan, R.J. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 39, 678–682 (2007).
Livingstone, M.S., Harris-Warrick, R.M. & Kravitz, E.A. Serotonin and octopamine produce opposite postures in lobsters. Science 208, 76–79 (1980).
Edwards, D.H. & Kravitz, E.A. Serotonin, social status and aggression. Curr. Opin. Neurobiol. 7, 812–819 (1997).
Stevenson, P.A., Hofmann, H.A., Schoch, K. & Schildberger, K. The fight and flight responses of crickets depleted of biogenic amines. J. Neurobiol. 43, 107–120 (2000).
Stevenson, P.A., Dyakonova, V., Rillich, J. & Schildberger, K. Octopamine and experience-dependent modulation of aggression in crickets. J. Neurosci. 25, 1431–1441 (2005).
Baier, A., Wittek, B. & Brembs, B. Drosophila as a new model organism for the neurobiology of aggression? J. Exp. Biol. 205, 1233–1240 (2002).
Certel, S.J., Savella, M.G., Schlegel, D.C. & Kravitz, E.A. Modulation of Drosophila male behavioral choice. Proc. Natl. Acad. Sci. USA 104, 4706–4711 (2007).
Hoyer, S.C. et al. Octopamine in male aggression of Drosophila. Curr. Biol. 18, 159–167 (2008).
Monastirioti, M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev. Biol. 264, 38–49 (2003).
Schwaerzel, M. et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10495–10502 (2003).
Valzelli, L. Aggressive behavior induced by isolation. in Aggressive Behavior (Eds. Garattini, S. and Sigg, E.B.) 70–76 (Wiley, New York, 1969).
Wang, L., Heiko Dankert, H., Perona, P. & Anderson, D.J. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc. Natl. Acad. Sci. USA 105, 5657–5663 (2008).
Chen, S., Lee, A.Y., Bowens, N.M., Huber, R. & Kravitz, E.A. Fighting fruit flies: a model system for the study of aggression. Proc. Natl. Acad. Sci. USA 99, 5664–5668 (2002).
Kitamoto, T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81–92 (2001).
Villella, A. et al. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics 147, 1107–1130 (1997).
Luan, H. et al. Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J. Neurosci. 26, 573–584 (2006).
Cole, S.H. et al. Two functional, but noncomplementing, Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 280, 14948–14955 (2005).
Kitamoto, T. Conditional disruption of synaptic transmission induces male-male courtship behavior in Drosophila. Proc. Natl. Acad. Sci. USA 99, 13232–13237 (2002).
Ganguly-Fitzgerald, I., Donlea, J. & Shaw, P.J. Waking experience affects sleep need in Drosophila. Science 313, 1775–1781 (2006).
Kravitz, E.A. Hormonal control of behavior: amines and the biasing of behavioural output in lobsters. Science 241, 1775–1781 (1988).
Adamo, S.A., Linn, C.E. & Hoy, R.R. The role of neurohormonal octopamine during 'fight or flight' behaviour in the field cricket Gryllus bimaculatus. J. Exp. Biol. 198, 1691–1700 (1995).
Roeder, T. Tyramine and octopamine: ruling behavior and metabolism. Annu. Rev. Entomol. 50, 447–477 (2005).
Sallinen, J., Haapalinna, A., Viitamaa, T., Kobilka, B.K. & Scheinin, M. Adrenergic α2C-receptors modulate the acoustic startle reflex, prepulse inhibition and aggression in mice. J. Neurosci. 18, 3035–3042 (1998).
Marino, M.D., Bourdelat-Parks, B.N., Cameron Liles, L. & Weinshenker, D. Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behav. Brain Res. 161, 197–203 (2005).
Bray, S. & Amrein, H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron 39, 1019–1029 (2003).
Svetec, N. & Ferveur, J.F. Social experience and pheromonal perception can change male-male interactions in Drosophila melanogaster. J. Exp. Biol. 208, 891–898 (2005).
Kulkarni, S.J. & Hall, J.C. Behavioral and cytogenetic analysis of the cacophony courtship song mutant and interacting genetic variants in Drosophila melanogaster. Genetics 115, 461–475 (1987).
Anholt, R.R., Lyman, R.F. & Mackay, T.F. Effects of single P-element insertions on olfactory behavior in Drosophila melanogaster. Genetics 143, 293–301 (1996).
Acknowledgements
We are grateful to M. Zhou and the Antibody Center of the National Institute of Biological Sciences for the antibody to TβH; J. Hirsh, M. Monastirioti, P. Shen, B. White, C.-F. Wu, R. Greenspan, P. Salvaterra, T. Kitamoto and the Bloomington Stock Center for fly stocks; X. Wu for participation in early experiments; Y. Jiang for help with statistics; X. Jia for technical assistance; and the Rao lab members for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 463 kb)
Supplementary Video 1
The chamber contains a wild-type fly painted red and a Tβhnm18 mutant fly painted yellow. The wild type often occupied the central food pad and chased after the Tβhnm18 mutant, preventing the latter from walking onto the food pad. The Tβhnm18 mutant was usually forced off and wandered along the wall of the fighting chamber. When the Tβhnm18 mutant had the chance of entering the territory with food, fighting was initiated and intensive lunging directed by the wild type towards the intruder Tβhnm18 mutant was often followed until the loser was forced off the food pad again. (MPG 2880 kb)
Rights and permissions
About this article
Cite this article
Zhou, C., Rao, Y. & Rao, Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci 11, 1059–1067 (2008). https://doi.org/10.1038/nn.2164
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2164